Abstract
Purpose
Spatially fractionated radiation therapy (SFRT) is a recognized technique for enhancing tumor response in radioresistant and bulky tumors. We analyzed clinical and treatment outcomes in patients with bone and soft tissue sarcomas treated with modern SFRT techniques.
Methods and Materials
Patients with metastatic or unresectable sarcoma treated with brass collimator, volumetric modulated arc therapy lattice, or proton SFRT from December 2019 to June 2022 were retrospectively reviewed. Consolidative external beam radiation therapy (EBRT) was delivered at the physician's discretion. Patient and treatment characteristics, treatment response (symptom improvement, local control, and imaging response), and toxicity data were collected.
Results
The cohort consisted of 53 patients treated with 61 SFRT treatments. Median age at treatment was 60.0 years. The primary location was soft tissue in 46 courses (75%) and bone in 15 (25%). Fifty-three courses (87%) were treated for symptom relief. The most used SFRT technique was volumetric modulated arc therapy lattice (n = 52, 85%) to a dose of 20 Gy (n = 48, 79%; range, 16-20 Gy). EBRT was delivered post-SFRT in 55 (90%) treatment courses with a median time interval from SFRT to EBRT of 5 days (range, 0-14 days). Median physical EBRT dose and fractionation was 40 Gy (range, 9-73.5 Gy) and 10 fractions (range, 3-33 fractions). Median follow up was 7.4 months (range, 0.2-30 months). One-year overall survival and local control rates were 53% and 82%. Symptom relief was documented with 32 treatment courses (60%). Stable or partial response was observed with 47 treatment courses (90%). Four grade 3 to 4 acute and subacute toxicities were attributable to SFRT (8%).
Conclusions
The current series is the largest to date documenting outcomes for SFRT in sarcomas. Our results suggest combined SFRT with EBRT is associated with a favorable toxicity profile and high rates of symptomatic and radiographic responses for metastatic or unresectable sarcomas.
Introduction
Sarcomas are a group of rare malignancies that comprise a heterogeneous mix of soft tissue and bone histologies. Radiation therapy (RT) can be a component of curative local treatment for sarcomas and may also be used as palliative treatment for metastatic disease. Historically, local tumor control with conventionally fractionated RT alone yields suboptimal results for most soft tissue and bone sarcomas. Sarcomas are extremely radioresistant and larger tumors are frequently not controlled. For instance, Kepka et al reported a 5-year local control rate of 45% in a series of patients with unresectable soft tissue sarcomas treated with definitive RT.1 Furthermore, the local control rate declined to 9% for tumors >10 cm.1 Similarly, RT alone has been associated with poor local tumor control for virtually all bone sarcoma histologies.2, 3, 4 Palliative RT is often used to address symptomatic sarcoma metastases. However, the percentage of patients who experience symptom relief is not well studied and current results are variable with series reporting improvements in 55% to 95% of treatment courses.5, 6, 7 As such, methods to improve tumor response and symptom relief for sarcoma patients treated with RT alone are needed.
Spatially fractionated radiation therapy (SFRT) is a technique that may enhance tumor response in radioresistant and bulky tumors.8,9 SFRT has been studied in certain disease sites and histologies, including head and neck cancers and melanoma, and demonstrated to correlate with acceptable disease control and toxicities.10, 11, 12 Currently, it is believed the radiobiology behind the response seen with SFRT includes the bystander effect (cells affected by RT communicate manifestations of damage to other cells not directly affected by RT), antitumor immune responses, and tumor microvasculature damage.9 SFRT may be beneficial in the treatment of advanced sarcomas given the radioresistant nature of these tumors, bulky tumor burden at time of treatment, and suboptimal local control rates seen despite RT dose-escalation.
A few case reports and small series have integrated SFRT therapy into the treatment paradigm for advanced sarcomas treated with RT and demonstrated improved clinical and pathologic response rates as well as symptom relief for these patients.13, 14, 15, 16, 17 This suggests SFRT therapy may be beneficial in optimizing the effectiveness of RT alone for advanced sarcomas and warrants further investigation. We analyzed clinical and treatment outcomes in 53 metastatic and/or unresectable sarcoma patients with 61 SFRT treatments, making this the largest series of sarcoma patients to date treated with modern SFRT techniques.
Methods and Materials
Patients
Sarcoma patients with metastatic disease or unresectable localized disease treated with SFRT from December 1, 2019, through June 1, 2022, at Mayo Clinic Rochester were retrospectively reviewed on an institutional review board–approved protocol. Use of SFRT for advanced sarcomas was at physician discretion. In general, SFRT was recommended when the intent of treatment was to elicit reduction in tumor size, provide durable local control, and/or provide symptom relief in tumors not amenable to stereotactic body radiation therapy. A total of 53 patients with 61 SFRT treatments were included in the analysis. Patients were treated with palliative intent in the setting of metastatic disease with the purpose of tumor response and symptom relief. Patients were treated with curative intent in the setting of localized, unresectable disease primarily to optimize local control.
SFRT therapy
All patients received SFRT as the sole therapy or before a course of consolidative external beam radiation therapy (EBRT). SFRT was delivered using brass, volumetric modulated arc therapy (VMAT) lattice, or proton therapy techniques as previously described by our institution, including dose to organs at risk.18, 19, 20 The recommended prescription dose was 16 to 20 Gy delivered in a single fraction based on the report from Mohiuddin et al that a higher response rate is seen with an SFRT dose of ≥15 Gy.8 In general, 20 Gy was administered whenever feasible. 16 to 18 Gy was administered when the tumor was in close proximity to normal tissues sensitive to RT (eg, bowel).
In general, brass GRID plans used a commercial brass block (.decimal) to deliver a single static field with either a 6 or 10 MV beam. The diameter of the holes at isocenter was 1.4 cm and were spaced 2.1 cm center-to-center in a hexagonal pattern. VMAT plans used 2 to 4 arcs and 6 MV flatting filter free beams. Spherical lattice points were placed throughout the physician contoured gross tumor volume (GTV) and optimized using 3 concentric ring structures to confine the highest doses to the center of the spheres and maximize dose sparing between the spheres. Sphere placement followed guidelines of a 1 to 1.5 cm sphere diameter, 2 to 3 cm sphere center-to-center separation, and sphere edge placement at least 1 cm away from any organ at risk. For proton SFRT, one field with a hexagonal spot scanning pattern was first applied. This was followed by a 2-step optimization process to reduce both the spot spacing and tubular shaped dose regions to a 1 cm radius.
Consolidative EBRT
Most patients received EBRT post-SFRT if they could clinically tolerate it and had not received prior irradiation in the same area. The prescription dose, fractionation scheme, and treatment modality of the EBRT course was at the discretion of the treating physician. In general, patients treated with palliative intent received a lower cumulative EBRT dose and shorter fractionation scheme whereas patients treated with curative intent treatment for unresectable localized disease received a higher EBRT dose (>50 Gy equivalent dose at 2 Gy per fraction) and longer fractionation scheme. Target doses were often limited due to bulky tumor volumes and proximity to critical tissues, such as bowel. Our institution's prior publication details the approach for dose constraints to organs at risk when combining SFRT with EBRT.20
Statistical methods
Data abstracted from the medical records included patient characteristics, tumor features, overall treatment paradigm, SFRT and EBRT treatment plan parameters, treatment-associated toxicities, imaging response, and oncologic outcomes. Imaging response was defined as stable disease (no change in tumor size, with or without tumor necrosis), partial response (any reduction in tumor size, with or without tumor necrosis), or progressive disease (any increase in tumor size and absence of tumor necrosis) at last follow-up imaging compared with pre-SFRT imaging. Tumor measurements were reported in 2- and 3-dimensions. Symptom relief was characterized by chart review of follow-up clinical notes documenting the change in presenting symptoms (eg, improved or no change). Toxicity was graded using Common Terminology Criteria for Adverse Events, version 5.
Descriptive statistics were used to collate patient and treatment characteristics as well as treatment response and toxicity. Kaplan-Meier analyses for local tumor control and overall survival were performed using BlueSky Statistics software version 10.3 (BlueSky Statistics LLC). Patients with <30 days of follow-up from SFRT and those without follow-up information were excluded from the treatment response and toxicity analyses.
Results
Patient characteristics
The cohort consisted of 53 patients with 61 SFRT treatments. Table 1 lists patient, tumor, and treatment characteristics. The median age at treatment was 60 years (range, 15-90 years). Site of origin was soft tissue in 46 courses (75%) and bone in 15 courses (25%). The most common soft tissue sarcoma and bone sarcoma histologies were leiomyosarcoma (n = 14, 23%) and chondrosarcoma (n = 7, 11%), respectively. Thorax (n = 18, 30%) was the most frequently treated site, followed by abdomen (n = 16, 26%) and pelvis (n = 16, 26%). Treatment intent was palliative in 51 treatment courses (82%). Treatment focused on the primary site of disease in most patients (n = 39, 64%). Symptoms were associated with 53 treatment courses (87%). The most common presenting symptom was pain (n = 40, 76%). Neurologic, gastrointestinal, pulmonary, and hematologic symptoms accounted for the remaining symptoms before treatment (n = 13, 25%).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristics | N (%) |
|---|---|
| All patients | 53 (100) |
| Treatment courses | 61 (100) |
| Median age (range), y | 60 (15-90) |
| Patient gender | |
| Male | 31 (57) |
| Female | 23 (43) |
| Primary location | |
| Soft tissue | 46 (75) |
| Bone | 15 (25) |
| Treatment site | |
| Thorax | 17 (30) |
| Pelvis | 16 (26) |
| Abdomen | 16 (26) |
| Extremity | 6 (10) |
| Axial | 5 (8) |
| Treatment site | |
| Primary | 39 (64) |
| Metastatic | 22 (36) |
| Treatment intent | |
| Curative | 10 (16) |
| Palliative | 51 (84) |
| Median SFRT GTV volume (range; IQR) | 636 cc (47-13, 373 cc; 1975 cc) |
| Median SFRT dose (range) | 20 Gy (16-20 Gy) |
| SFRT technique | |
| VMAT lattice | 52 (85) |
| Brass | 6 (10) |
| Proton therapy | 3 (5) |
| Consolidative EBRT | |
| Yes | 55 (90) |
| No | 6 (10) |
| Median EBRT dose (range; IQR) | 40 Gy (9-73.5 Gy; 15 Gy) |
| Median EBRT fractions (range; IQR) | 10 fx (3-33 fx; 5 fx) |
| Median EBRT EQD2 (range; IQR) | 43 Gy (11-83 Gy; 19 Gy) |
| Median EBRT BED10 (range; IQR) | 56 Gy (12-92 Gy; 22 Gy) |
| Median number of days between SFRT and EBRT (range) | 5 d (range, 0-14 d) |
| EBRT modality | |
| IMRT | 43 (78) |
| Proton therapy | 11 (20) |
| IMRT and proton therapy | 1 (2) |
Abbreviations: BED10 = biologically equivalent dose; EBRT = external beam radiation therapy; EQD2 = equivalent dose at 2 Gy per fraction; GTV = gross tumor volume; IMRT = intensity modulated radiation therapy; SFRT = spatially fractionated radiation therapy; VMAT = volumetric modulated arc therapy.
Dosimetric features of SFRT plans
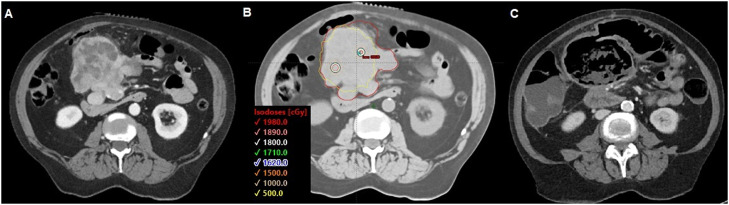
Median GTV volume for SFRT was 636 cc (range, 47-13,373 cc; IQR, 1975 cc). Two patients did not have a GTV volume for SFRT contoured. In addition, 20 Gy was the most used SFRT dose (n = 48, 79%; range, 16-20 Gy). Most courses were treated with a VMAT lattice technique (n = 52, 85%). Brass and proton GRID were used in 6 (10%) and 3 (5%) courses, respectively. Figure 1 shows representative brass, VMAT, and proton SFRT plans from the cohort.
Figure 1.
(A) Brass spatially fractionated radiation therapy plan for 26-year-old woman with malignant phyllodes of the chest-wall. (B) volumetric modulated arc therapy lattice plan 55-year-old woman with abdominal gastrointestinal stromal tumor (GIST). (C) Proton spatially fractionated radiation therapy plan for 56-year-old woman with pelvic osteosarcoma. All plans were treated to 20 Gy.
EBRT was delivered post-SFRT in 55 (90%) treatment courses with a median time interval from SFRT of 5 days (range, 0-14 days). Intensity modulated radiation therapy was used in 43 courses (78%), proton therapy in 11 courses (20%), and combination intensity modulated radiation therapy and proton therapy in 1 course (2%). Median EBRT dose and fractionation was 40 Gy (range, 9-73.5 Gy; IQR, 15 Gy) and 10 fractions (range, 3-33 fractions; IQR, 5 fractions), respectively. With an α /β of 4, this equates to a median equivalent dose at 2 Gy per fraction dose of 43 Gy (range, 11-83; IQR range, 19 Gy). Median BED was 56 Gy10 (range, 12-92; IQR, 22 Gy). Concurrent systemic therapy was administered in 5 treatment courses (9%), and 1 patient underwent tumor gross total resection 2 months after GRID-only treatment for a chest-wall desmoid.
Response and survival
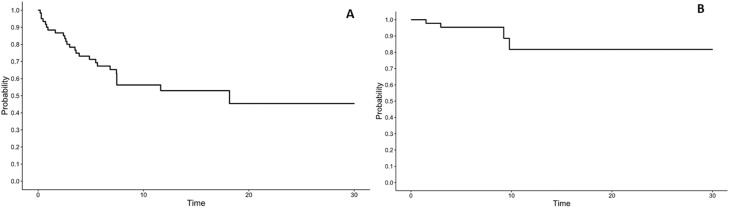
Median follow-up was 7.4 months (range, 0.2-30 months). At last follow-up, 22 patients were dead of disease (42%), 27 patients were alive with disease (51%), and 4 patients were alive with no evidence of active/metastatic disease (8%). The 1-year overall-survival rate was 53.0% (95% CI, 41%-69%; Fig. 2A). Eight treatment courses were excluded from the treatment-associated toxicity and clinical and imaging response analyses because these patients expired within 1 month of finishing RT. An additional 2 patients were excluded as they expired before posttreatment imaging and follow-up was obtained, resulting in 10 treatment courses not evaluable for imaging and clinical response. Median time to imaging from SFRT treatment was 6 months (range, 1-30; IQR 7). Stable imaging response was observed with 18 treatment courses (35%), partial response with 29 treatment courses (56%; Fig. 3), and progressive disease with 5 treatment courses (10%). The 1-year local control rate was 82% (95% CI, 65%-100%; Fig. 2B). Median time to disease progression was 6 months (range, 1-10). Median duration of response in those who did not have disease progression was 6 months (range, 1-30). Symptom relief was documented with 32 treatment courses (60%). Median time to symptom relief from treatment was 1.6 months (range, 1 day to 15.6 months). The 1-year distant failure rate was 42% (95% CI, 29%-60%).
Figure 2.
Kaplan-Meier estimates for (A) overall survival (n = 53 patients) and (B) local control (n = 51 treatment courses).
Figure 3.
Treatment course for a 39-year-old woman with synovial sarcoma metastasis treated with 20 Gy spatially fractionated radiation therapy plus 40 Gy in 10 fractions external beam radiation therapy. (A) Pelvic computed tomography demonstrating disease extent at time of radiation planning. (B) Spatially fractionated radiation therapy treatment at same axial level. Gross tumor volume delineated in red. (C) Pelvic computed tomography demonstrating tumor response at same axial level, 1 month later.
In the 4 patients with local tumor progression, tumor involved the extremity in one patient and abdomen-pelvis in 3 patients. All patients were treated with palliative intent and had a soft tissue sarcoma histology. One patient was treated with brass SFRT, and the remaining were treated with VMAT SFRT. SFRT dose was 18 Gy in 2 patients and 20 Gy in 2 patients. Two patients did not receive EBRT. EBRT dose was 40 Gy in 10 fractions for both patients. Three patients did not experience symptom relief and 1 patient did experience symptom relief. One patient died of disease.
Seven patients associated with 8 treatment courses died within 1 month of finishing RT. Median patient age for this cohort was 63 years (range, 15-72). Treatment intent was palliative for all courses with 4 courses treated at a primary site (50%). Seven treatment courses (88%) were delivered for pain symptoms. Site of origin was soft tissue in 6 courses (75%); and primary tumor site was pelvis in 4 (50%), extremity in 2 (25%), abdomen in one (13%), and thorax in one (13%). Consolidative EBRT was delivered with 5 courses (63%). None of the courses were administered with concurrent or subsequent systemic or surgical therapies.
Toxicity
Treatment-associated toxicities are listed in Table 2. No treatment related toxicities were documented in 45 treatment courses (74%) and grade 1 to 2 toxicities were observed in 12 treatment courses (20%). Four grade 3 to 4 acute and subacute toxicities were attributable to SFRT (8%). These cases included grade 3 radiation dermatitis involving the inguinal fold for a palliative extremity sarcoma treatment, grade 3 radiation pneumonitis treated with steroids for a palliative lung sarcoma treatment, grade 3 large bowel obstruction requiring colostomy for a palliative pelvic sarcoma treatment, and grade 4 bowel fistula (Fig. 4) requiring surgical exploration for a palliative abdominal sarcoma treatment.
Table 2.
Treatment associated toxicities (n = 61 treatment sites)
| CTCAE grade |
|||||
|---|---|---|---|---|---|
| Toxicities | 1 | 2 | 3 | 4 | 5 |
| Skin | 1 | 1 | |||
| Fatigue | 5 | 1 | |||
| Gastrointestinal* | 5 | 1 | 1 | 1 | |
| Urinary retention | 1 | ||||
| Bone fracture | 2 | ||||
| Pneumonitis | 1 | 1 | |||
| Mucositis | 1 | ||||
| Dysgeusia | 1 | ||||
| Dysphagia | 1 | ||||
| Dyspnea | 1 | ||||
| Pain | 1 | 1 | |||
Includes nausea, diarrhea, enteritis, bowel obstruction, and fistula.
Abbreviation: CTCAE = Common Terminology Criteria for Adverse Events, version 5.
Figure 4.
Treatment course for a 60-year-old woman with abdominal leiomyosarcoma metastasis treated with 18 Gy spatially fractionated radiation therapy plus 35 Gy in 10 fractions external beam radiation therapy. (A) Abdominal computed tomography demonstrating disease extent at time of radiation planning. (B) Spatially fractionated radiation therapy treatment at same axial level. Gross tumor volume delineated in red. (C) Abdominal computed tomography demonstrating tumor response and fistula toxicity at same axial level, 8 months later.
Evaluation of radiation plans for the 4 grade 3 to 4 toxicities revealed the following. For the case of grade 4 bowel fistula, tumor was intertwined with bowel and a SFRT VMAT sphere was placed in the bowel, resulting in the fistula. SFRT and EBRT doses were 18 Gy and 35 Gy/10 fractions, respectively. Maximum small dose on the sum plan was 42 Gy. In the case of grade 3 bowel obstruction, SFRT and EBRT doses were 20 Gy and 25 Gy/5 fractions, respectively. Maximum large bowel dose on the sum plan was 29 Gy. The obstruction was likely a result of tumor aggravated by RT. In the case with grade 3 dermatitis, the SFRT and EBRT doses were 20 Gy and 40 Gy/10 fractions, respectively. Maximum skin dose on the sum plan was 42 Gy. Finally, for the case of grade 3 radiation pneumonitis, the SFRT and EBRT doses were 20 Gy and 40 Gy/10 fractions, respectively. Mean total lung dose in the sum plan was 10 Gy.
Concurrent systemic therapy was administered in 2 patients who experienced treatment-associated toxicities. Eribulin was administered in the palliative extremity patient who experienced grade 3 radiation dermatitis, and temozolomide and irinotecan was administered in a palliative pelvis patient who experienced grade 1 diarrhea.
Discussion
Patients who received a diagnosis of metastatic or unresectable sarcomas experience significant morbidity and mortality.21,22 Sarcomas are extremely radioresistant and when they are bulky, durable local control is often difficult to achieve. SFRT is a treatment approach designed to enhance the therapeutic response to EBRT in radioresistant and bulky tumors. We report the largest series to date of metastatic or unresectable sarcomas treated with modern SFRT techniques and demonstrate that SFRT is associated with a favorable toxicity profile (20% grades 1-2; 8% grades 3-4) as well as high rates of symptomatic (60.4%) and radiographic responses (90% stable disease or partial response).
SFRT for the treatment of bulky tumors has a history of over 100 years, though initially delivered with orthovoltage x-rays.9 The introduction of megavoltage x-rays, skin sparing techniques, and superior dosimetry with treatment planning platforms led to SFRT falling out of favor in clinical practice.9 However, modern advances in RT physics and technology have provided more techniques with which to enhance SFRT dosimetry and delivery, resulting in a resurgence of SFRT investigations.9 Sarcomas are traditionally considered to be radioresistant tumors given the often larger tumor sizes at time of treatment, higher doses needed for therapeutic effect, and suboptimal response rates despite RT dose-escalation. For example, Kepka et al evaluated local control in a series of patients with unresectable soft tissue sarcomas treated with definitive RT and reported the local control rate declined to 0% for tumors greater than 10 cm treated to <63 Gy.1 Given the often-large tumor sizes for advanced sarcomas, stereotactic body radiation therapy/stereotactic radiosurgery are unsuitable treatment approaches. As such, SFRT is gaining popularity in the treatment of metastatic and/or unresectable sarcomas.
The modern use of SFRT for the treatment of advanced sarcomas has mostly been limited to case reports and small series thus far.13, 14, 15, 16, 17 Nevertheless, these reports demonstrate improved response rates for patients treated with SFRT.13, 14, 15, 16, 17,23 Kaiser et al reported the case of an 82-year-old woman with a spindle cell sarcoma of the upper extremity measuring 926 cm3 volumetrically treated with 18 Gy SFRT followed by 32 Gy in 11 fractions EBRT.14 The tumor responded dramatically with only necrotic debris present before surgery.14 Pathology from the margin negative resection demonstrated 65 cm3 of residual tumor and 5% to 10% viable cells.14 The high percentage of necrosis was unexpected given that the tumor was high-grade, received less than the standard dose of 50 Gy for preoperative RT, and neoadjuvant chemotherapy was not administered. Roberge et al documented a median pathologic treatment response of 50% for high-grade sarcomas treated with conventionally fractionated 50 Gy preoperative RT only on retrospective review, and RTOG0630 documented a pathologic complete response rate of 19.4% in extremity soft tissue sarcomas with a median tumor size of 10.5 cm treated with 50 Gy in 25 fractions preoperative RT ± chemotherapy.23,24 Snider et al delivered 15 Gy SFRT followed by 45 to 50.4 Gy EBRT to 26 patients with bulky (>8 cm) osteosarcomas and soft tissue sarcomas before surgery.13 A pathologic complete response was observed in 35.3% of all high-grade sarcomas, 50% of high-grade sarcomas involving the extremities, and 50% of osteosarcomas. The value of pathologic complete response rates in sarcoma as an oncologic endpoint is debated and warrants further investigation in the context of SFRT.
We elected to incorporate SFRT into our practice for metastatic and/or unresectable sarcomas given the associated symptoms and morbidity with these advanced cases as well as the suboptimal response rates and local control with palliative RT.22,25 For instance, Boyce-Fappiano et al reported a best response rate in 70 of 73 patients (96%) with unresectable and/or metastatic sarcomas treated with hypofractionated (>10 fractions) RT.7 Mohiuddin et al used SFRT ± EBRT in 33 patients (44 treatment sites) with recurrent or unresectable soft tissue sarcomas.15 The median SFRT and EBRT doses were 15 Gy and 50 Gy, respectively.15 Stable disease or some degree of response was noted in 80% of patients.15 We report similar response results in our cohort with 90% of tumors documented as stable disease or experiencing a partial radiographic response. We are the first in the literature to report percentage of patients with symptom improvement after SFRT for advanced sarcomas. Approximately 87% of treatment courses were delivered to palliate tumor-related symptoms and 60% had subjective symptom improvement. Our results fall within the range of symptom improvement rates reported in previously published palliative RT series for advanced sarcomas. For example, Tween et al reported a 70% symptom response rate to palliative RT in soft tissue sarcomas, and a 55% response rate in bone sarcomas.5 The limitation of these analyses, including ours, is the retrospective nature of chart reviews which may underreport symptom improvement rates. Nevertheless, our results suggest SFRT with or without EBRT can be associated with high rates of symptom relief for patients with advanced sarcomas and warrants further investigation and data collection.
Our series also demonstrates that SFRT is associated with a favorable toxicity profile. Grades 1 to 2 toxicities were noted in 20% and grades 3 to 4 toxicities were documented in 8% of the cohort. Other SFRT series have also reported favorable toxicity profiles.13, 14, 15, 16, 17 Mohiuddin et al reported 2 patients who experienced grade 3 skin reaction in the analysis of 33 patients with recurrent and/or unresectable soft tissue sarcomas.15 In our series, one patient experienced a grade 4 bowel fistula requiring surgical exploration (Fig. 4). The tumor was intertwined with the bowel, and on retrospective review it was evident that one of the VMAT SFRT spheres was placed in bowel and this likely resulted in the fistula toxicity. After this event, our simulation, contouring, and treatment planning practices evolved significantly to ensure safer delivery of SFRT.20 For example, we now routinely use intravenous and oral contrast for abdominal and pelvic tumors to delineate bowel. We also developed standardized approaches to VMAT SFRT sphere placement, dose optimization, plan evaluation, and constraints for organs at risk.20 With these standardized practice implementations, we believe our SFRT practice is now safer, and we will continue to collect and report data regarding the safety and efficacy of SFRT for advanced sarcomas.
Many questions remain unanswered regarding the optimal delivery of SFRT with or without EBRT for advanced sarcomas. This includes whether EBRT is needed for effective palliation after SFRT, the optimal EBRT dose and fractionation, and time between the SFRT and EBRT courses. The median EBRT dose and fraction number in our series was 40 Gy and 10 fractions, respectively. The median number of days between SFRT and EBRT was 5 days. Most other published reports combined SFRT with EBRT (variable dose and fractionation schemes) and reported a variable period between the 2 treatments.14, 15, 16 For instance, Snider et al noted a break of 2 to 3 days was “generally preferred” between SFRT and the start of preoperative radiation therapy (45-50.4 Gy conventionally fractionated) for their cohort of high risk soft tissue sarcoma and osteosarcoma.13 Most patients in our series received EBRT and as such we cannot confidently determine which patients are most likely to benefit from SFRT alone. Further understanding of the radiobiology behind SFRT may also help answer some of these questions.
Currently, it is hypothesized that the bystander effect, vascular damage, and antitumor immune responses may play a role in the mechanism of SFRT efficacy based on preclinical data.9,26 Ongoing research efforts to understand the cellular environment and processes in correlation with clinical outcomes for SFRT will be helpful in determining the optimal treatment approach (NCT01967927, NCT04549246, clinicaltrials.gov). It is also important to note that 7 patients in our cohort expired within 1 month of finishing RT. This highlights the need to evaluate a patient's performance status, select shorter EBRT courses, and to develop patient selection criteria for those most likely to derive the benefits of durable symptom improvement and/or local control.
This study has many limitations including its retrospective nature, especially as it relates to symptom and imaging response data collection and analyses. Nevertheless, our results suggest SFRT with EBRT may be a useful treatment approach to provide symptom relief and achieve a tumor response in unresectable or metastatic sarcomas. We favor this approach for large, advanced sarcomas. A shorter course of EBRT is likely beneficial in patients anticipated to have a very short life expectancy. We plan to open a prospective study designed to evaluate the optimal use of EBRT with or without SFRT, as well as methodically evaluate for symptom relief and tumor response, in advanced sarcomas as the next step.
Conclusion
Our study demonstrates modern era SFRT is an innovative, feasible, and safe treatment strategy for metastatic and unresectable sarcomas. Combined SFRT with hypofractionated EBRT is associated with a favorable toxicity profile and high rates of symptomatic and radiographic responses. Survival is generally poor for this population indicating a shorter course of EBRT is preferred, which also permits patients with other sites of disease to resume systemic therapy more quickly. Future studies will need to better identify patients with sarcoma who are most likely to benefit from EBRT with or without SFRT.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sources of support: This work had no specific funding.
References
- 1.Kepka L, DeLaney TF, Suit HD, Goldberg SI. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63:852–859. doi: 10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SK, Randall RL, DuBois SG, et al. Identification of patients with localized ewing sarcoma at higher risk for local failure: A report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2017;99:1286–1294. doi: 10.1016/j.ijrobp.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz R, Bruland O, Cassoni A, Schomberg P, Bielack S. The role of radiotherapy in oseosarcoma. Cancer Treat Res. 2009;152:147–164. doi: 10.1007/978-1-4419-0284-9_7. [DOI] [PubMed] [Google Scholar]
- 4.De Amorim Bernstein K, DeLaney T. Chordomas and chondrosarcomas: The role of radiation therapy. J Surg Oncol. 2016;114:564–569. doi: 10.1002/jso.24368. [DOI] [PubMed] [Google Scholar]
- 5.Tween H, Peake D, Spooner D, Sherriff J. Radiotherapy for the palliation of advanced sarcomas: The effectiveness of radiotherapy in providing symptomatic improvement for advanced sarcomas in a single centre cohort. Healthcare (Basel) 2019;7:120. doi: 10.3390/healthcare7040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soyfer V, Corn BW, Kollender Y, Tempelhoff H, Meller I, Merimsky O. Radiation therapy for palliation of sarcoma metastases: A unique and uniform hypofractionation experience. Sarcoma. 2010;2010 doi: 10.1155/2010/927972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyce-Fappiano D, Damron EP, Farooqi A, et al. Hypofractionated radiation therapy for unresectable or metastatic sarcoma lesions. Adv Radiat Oncol. 2022;7 doi: 10.1016/j.adro.2022.100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohiuddin M, Fujita M, Regine WF, Megooni AS, Ibbott GS, Ahmed MM. High-dose spatially-fractionated radiation (GRID): A new paradigm in the management of advanced cancers. Int J Radiat Oncol Biol Phys. 1999;45:721–727. doi: 10.1016/s0360-3016(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 9.Yan W, Khan KK, Wu X, et al. Spatially fractionated radiation therapy: History, present and the future. Clin Transl Radiat Oncol. 2019;20:30–38. doi: 10.1016/j.ctro.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huhn JL, Regine WF, Valentino JP, Meigooni AS, Kudrimoti M, Mohiuddin M. Spatially fractionated GRID radiation treatment of advanced neck disease associated with head and neck cancer. Technol Cancer Res Treat. 2006;5:607–612. doi: 10.1177/153303460600500608. [DOI] [PubMed] [Google Scholar]
- 11.Peñagarícano JA, Moros EG, Ratanatharathorn V, Yan Y, Corry P. Evaluation of spatially fractionated radiotherapy (GRID) and definitive chemoradiotherapy with curative intent for locally advanced squamous cell carcinoma of the head and neck: Initial response rates and toxicity. Int J Radiat Oncol Biol Phys. 2010;76:1369–1375. doi: 10.1016/j.ijrobp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Sathishkumar S, Dey S, Meigooni AS, et al. The impact of TNF-alpha induction on therapeutic efficacy following high dose spatially fractionated (GRID) radiation. Technol Cancer Res Treat. 2002;1:141–147. doi: 10.1177/153303460200100207. [DOI] [PubMed] [Google Scholar]
- 13.Snider JW, Molitoris J, Shyu S, et al. Spatially fractionated radiotherapy (GRID) prior to standard neoadjuvant conventionally fractionated radiotherapy for bulky, high-risk soft tissue and osteosarcomas: Feasibility, safety, and promising pathologic response rates. Radiat Res. 2020;194:707–714. doi: 10.1667/RADE-20-00100.1. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser A, Mohiuddin M, Jackson G. Dramatic response from neoadjuvant, spatially fractionated GRID radiotherapy (SFGRT) for large, high-grade extremity sarcoma. J Radiat Oncol. 2013;2:103–106. [Google Scholar]
- 15.Mohiuddin M, Miller T, Ronjon P, Malik U. Spatially fractionated grid radiation (SFGRT): A novel approach in the management of recurrent and unresectable soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2009;75:S526. [Google Scholar]
- 16.Mohiuddin M, Memon M, Nobah A, et al. Locally advanced high-grade extremity soft tissue sarcoma: Response with novel approach to neoadjuvant chemoradiation using induction spatially fractionated GRID radiotherapy (SFGRT) J Clin Oncol. 2014;32:10575. [Google Scholar]
- 17.Kudrimoti M, Mohiuddin M, Ahmed MM, et al. Use of high dose spatially fractionated radiation (GRID therapy) in management of large, poor prognostic stage III (>10 cms) soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2004;60:S575. [Google Scholar]
- 18.Grams MP, Owen D, Park SS, et al. VMAT Grid therapy: A widely applicable planning approach. Pract Radiat Oncol. 2021;11:e339–e347. doi: 10.1016/j.prro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Grams MP, Tseung H, Ito S, et al. A Dosimetric comparison of lattice, brass, and proton grid therapy treatment plans. Pract Radiat Oncol. 2022;12:e442–e452. doi: 10.1016/j.prro.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Grams MP, Deufel CL, Kavanaugh JA, et al. Clinical aspects of spatially fractionated radiation therapy treatments. Phys Med. 2023;111:102616. doi: 10.1016/j.ejmp.2023.102616. [DOI] [PubMed] [Google Scholar]
- 21.Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–1054. doi: 10.1002/cncr.25538. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Matsumine A, Matsubara T, et al. Retrospective analysis of metastatic sarcoma patients. Oncol Lett. 2011;2:315–318. doi: 10.3892/ol.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberge D, Skamene T, Nahal A, Turcotte RE, Powell T, Freeman C. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97:404–407. doi: 10.1016/j.radonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Zhang Q, Eisenberg BL, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015;33:2231–2238. doi: 10.1200/JCO.2014.58.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandra R, Hwang N, Matharu GS, Reddy K, Grimer R. One-year mortality in patients with bone and soft tissue sarcomas as an indicator of delay in presentation. Ann R Coll Surg Engl. 2015;97:425–433. doi: 10.1308/003588415X14181254790284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song CW, Terezakis S, Park W-Y, et al. Preferential tumor vascular damage is the common antitumor mechanism of high-dose hypofractionated radiation therapy: SABR, spatially fractionated radiation therapy, and FLASH radiation therapy. Int J Radiat Oncol Biol Phys. 2023;117:701–704. doi: 10.1016/j.ijrobp.2023.05.015. [DOI] [PubMed] [Google Scholar]