Highlights
-
•
T-2 and its six modified forms were simultaneously detected by UPLC/MS-MS for the first time.
-
•
T-2 and its modified forms co-occurred in cereals and cereal-based products from Shanghai.
-
•
Exposure assessment results indicated T-2 and its modified forms could lead to health risk.
Keywords: T-2 toxin, Modified mycotoxins, Trichothecenes, UPLC-MS/MS, Cereals and cereal-based products, Dietary exposure assessment
Abstract
A reliable and sensitive UPLC-MS/MS method coupled with HLB-SPE was developed for simultaneous determination of T-2 and its modified forms (HT-2, NEO, T-2-triol, T-2-tetraol, T-2-3G, and HT-2-3G) in cereals and cereal-based products. Acceptable linearity (R2 ≥ 0.99), limits of quantitation (0.5–10.0 μg/kg), intra-day precision (RSD < 12.8 %), inter-day precision (RSD ≤ 15.8 %), and recovery (76.8 %-115.2 %) were obtained for all analytes in all matrices investigated. 107 commercial foodstuffs were analyzed, and T-2 was detected in 29.0 % of maize and maize flour samples (0.51 to 56.61 μg/kg) and in 10–33.3 % of wheat flour and barley samples (1.27 to 78.51 μg/kg). Moreover, 66.7 % of the positive samples were simultaneously contaminated with two or more T-2 forms. The possible health risk related to T-2 and its modified forms in cereals and cereal-based products was evaluated using a probabilistic dietary exposure assessment. The 95th percentile dietary exposure values of the sum of T-2 forms ranged from 0.16 to 1.70 ng/kg b.w./day for lower bound (LB), and 0.17 to 7.59 ng/kg b.w./day for upper bound (UB). Results strongly suggested that the presence of T-2 and its modified forms in cereals and cereal-based products warrants greater attention and investigation, although probabilistic dietary exposure values currently remain below the tolerable daily intake (TDI) value of 20 ng/kg b.w./day.
1. Introduction
T-2 toxin (T-2), the most important member of type A group trichothecenes, is mainly produced by Fusarium tricinctum, Fusarium sporotrichioides, and Fusarium poae found in cereals and cereal-based foods (Kochiieru et al., 2020, Mankevičienė et al., 2006). Both acute and chronic expose of T-2 are associated with various health issues including anorexia, vomiting, dermal toxicity, immunotoxicity, nephrotoxicity, hepatotoxicity, and teratogenicity (Chen et al., 2020, Taroncher et al., 2021, Yang et al., 2020, Yang et al., 2020, Zhang et al., 2021). During the growth of fungi and plants and when ingested by mammals, T-2 could be transformed to various modified forms. One or more of the three ester groups of T-2 are easily hydrolyzed to produce the HT-2 toxin (HT-2), neosolaniol (NEO), T-2-triol, or T-2-tetraol, which are considered the predominant modified forms of T-2. Other modified forms of T-2 are usually formed through conjugation to glucose, sulfate or acetyl groups, and are also known as “masked mycotoxins”, mainly including T-2-3-glucoside (T-2-3G) and HT-2-3-glucoside (HT-2-3G) (McCormick et al., 2015, Wu et al., 2020; S. Yang, Poucke, Wang, Zhang, Saeger, & Boevre, 2017). It should be noted that the modified forms of T-2 are generally considered to have equivalent or only slightly reduced toxicity due to well-retained toxic structures and the possibility of hydrolization back to T-2 (Kasimir et al., 2020, Ling et al., 2020). The structures of T-2 toxin and its modified forms are shown in Fig. 1.
Fig. 1.
Chemical structures of T-2 toxin and its modified forms.
Cereal and cereal-based products are an indispensable component of human diet and are susceptible to T-2/HT-2 contamination in the field and during processing and storage, especially for oat, barley, wheat, maize (Pleadin, Vasilj, Kudumija, Petrović, Vilušić, & Škrivanko, 2017). Based on 240 cereal samples, T-2/HT-2 contamination was widespread, found in 70.0 % (oats), 40.9 % (barley), 26.8 % (maize) and 19.2 % (wheat) of the samples, with sum concentrations ranging from 12.2 to 332.3 µg/kg (Kiš, Vuli, Kudumija, Arkanj, & Pleadin, 2021). To protect consumer health, the European Food Safety Authority (EFSA) reduced the tolerable daily intake (TDI) for the sum of T-2 and HT-2 to 0.02 μg/kg b.w./day, only one fifth of its initial established TDI (0.1 μg/kg b.w./day). An acute reference dose (ARfD) for T-2 or HT-2 was also set as 0.3 μg/kg b.w./day (EFSA, 2011, EFSA, 2017). Many countries have imposed regulations on the maximum levels in cereals and cereal-products ranging from 100 to 2000 μg/kg (Commission Recommendation, 2013). However, the maximum levels for T-2 and HT-2 in China are only applicable to feed.
In recent decades, numerous analytical methods, i.e., gas chromatography-mass spectrometer (GC–MS) (Pereira, Fernandes, & Cunha, 2015), high-performance liquid chromatography (HPLC) fluorescence (FLR) detectors (National Standard of the People's Republic of China), and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (Gab-Allah, Tahoun, Yamani, Rend, & Shehata, 2022), have been established for detection of T-2 and HT-2 in cereals and cereal-based products. Among them, HPLC-MS/MS methodology is usually favored due to its high sensitivity, simple sample preparation and wide compatibility for different compound polarities. However, most of the previous reports only focused on T-2 and HT-2, and their important modified forms (e.g., T-2-triol, T-2-tetraol, T-2-3G, and HT-2-3G) were usually ignored (Nathanail et al., 2015, Nathanail et al., 2015, Ostry et al., 2017). Liquid chromatography high-resolution mass spectrometry (LC-HRMS) based methods are frequently used only as fully qualitative or semi-quantitative method for screening and identification of the modified forms of T-2 in the sporadic studies, which are also lack of fully validated methodology (McCormick et al., 2012, Meng-Reiterer et al., 2015, Nathanail et al., 2015, Nathanail et al., 2015). The few data available on the occurrence of these modified forms indicate that they are primarily found in cereal-based products. Drakopoulos has reported that T-2-tetraol and HT-2-3G were present in more than 2 % of barley samples with the mean concentration of 75 and 18 μg/kg, respectively (Drakopoulos, Sulyok, Krska, Logrieco, & Vogelgsang, 2021). Similarly, T-2-3G and HT-2-3G were identified and characterized in contaminated barley, wheat, and oat samples.
Considering the adverse health effects, it is of great importance to investigate the dietary exposure to T-2 and its modified forms. However, to our knowledge, the present assessments of dietary exposure or risk assessments were only focused on T-2 and HT-2, and other important modified forms of T-2 were not investigated, which lead to the lack of sufficient occurrence data and the exposure of human population to these mycotoxins. Therefore, the objective of presented study is to 1) establish and validate an accurate and sensitive UPLC-MS/MS method for the simultaneous determination of T-2 and its six modified forms; 2) conduct a comprehensively screening for the occurrence of these mycotoxins in commercial cereals and cereal-based products collected from Shanghai; 3) assess the dietary exposure of Shanghai adult population to T2 and its six modified forms by a probabilistic evaluation. This work is expected to provide a novel and reliable strategy for the screening and dietary exposure assessment of T-2 and its modified forms, which could not only provide more comprehensive occurrence data, but also accurately evaluate the health risk to population from modified mycotoxins.
2. Materials and methods
2.1. Chemicals and regents
Reference materials of T-2 (100.6 μg/mL), HT-2 (100.1 μg/mL), and NEO (100.4 μg/mL) were purchased from Romer Labs (Tulln, Austria). T-2-triol (1 mg) and T-2-tetraol (1 mg) were purchased from Pribolab (Qingdao, China). T-2-3G and HT-2-3G were prepared and confirmed in our laboratory based on methods in previous publication (McCormick et al., 2015). Hydrophile Lipophilic Balance (HLB) SPE cartridges filling the polystyrene divinylphenyl pyrrolidone were obtained from GuangPuDa Co. (Beijing, China). Methanol and acetonitrile, both HPLC grade, were provided by Merck (Darmstadt, Germany). Ammonium acetate (HPLC grade) was procured from Anpel (Shanghai, China). Ultra-pure-grade water, used throughout the whole analysis, was produced by a Milli-Q water purification system (Millipore, MA, USA).
2.2. Samples
A total of 107 cereals and cereal-based products (20 maize samples, 11 maize flour samples, 12 wheat samples, 20 wheat flour samples, 20 rice samples, 12 sorghum samples, 4 barley samples and 8 oatmeal samples), approximately 500 g of each, were randomly selected from supermarkets and farmers' markets in various regions of Shanghai (Table S1). All samples were ground with a high-speed mill and allowed to pass through a 50 mesh sieve. Then the samples were vacuumed packaged with aluminum foil bags and stored at 4 °C until analysis.
2.3. Sample preparation
Ground samples (2.0 g) were weighed into a 50 mL centrifuge tube, to which 10 mL of acetonitrile/water solution (84:16, v/v) was added. The mixture was extracted for 30 min using a rotary shaker and centrifuged for 5 min at 4500 rpm. Subsequently, 5 mL of the supernatant was evaporated to dryness under nitrogen gas at 45 °C. The residues were reconstituted in 5 mL of methanol/water solution (5:95, v/v) and passed through a HLB cartridge, which were pre-conditioned with 2 mL methanol and 2 mL water. The cartridges were then washed with 5 mL water and eluted with 5 mL methanol. After drying under nitrogen gas at 45 °C, the eluent was re-dissolved in 1 mL of acetonitrile/water (20/80, v/v), passed through a 0.22 μm nylon filter and then ready for UPLC-MS/MS analysis.
2.4. UPLC-MS/MS analysis
UPLC-MS/MS analysis was performed on a Waters ACQUITY UPLC system coupled with a Waters XEVO TQ-S mass spectrometer (Waters, Milford, MA, USA). Chromatographic separation was achieved on a Waters XBridge® BEH C18 column (3.0 × 100 mm, 2.5 μm) with acetonitrile (eluent A) and aqueous solution of 5 mmol/L ammonium acetate (eluent B) during the mobile phase. A linear gradient elution program was applied and the proportion of eluent A was increased from 10 % to 90 % in 5 min, kept constant for 1 min, and the column was then re-equilibrated with 10 % eluent B for 2 min, giving a total run time of 8 min. The flow rate, column temperature, and injection volume were set as 0.4 mL/min, 40 °C, and 3 μL, respectively. For MS/MS analysis, both positive electrospray ionization mode (ESI+) and negative electrospray ionization mode (ESI−) of the electrospray ionization source were used with the following parameters: interface voltages of capillary, 2.5 kV (ESI+) and 1.5 kV (ESI−); voltages of cone: 25 V (ESI+) and 20 V (ESI−); desolvation temperature, 500 °C; source temperature, 150 °C; the gas of cone and desolvation was nitrogen and the flow rates were set as 55 and 1000 L/h, respectively. The collision gas was argon and the flow rate was set as 0.5 L/h. The detailed multiple reaction monitoring (MRM) parameters of each mycotoxin were listed in Table 1.
Table 1.
Mass parameters for T-2 toxin and its modified forms.
| Mycotoxins | Formula | Molecular Weight | Precursor ions (m/z) | Product ions (m/z) | CE (V) | Cone (V) |
|---|---|---|---|---|---|---|
| NEO | C19H26O8 | 382.4 | 400.4 [M + NH4]+ | 305.2*/185.1 | 12/20 | 30 |
| T-2-tetraol | C15H22O6 | 298.3 | 297.2 [M - H]− | 219.1*/201.1 | 8/15 | 20 |
| T-2-triol | C20H30O7 | 382.5 | 405.2 [M + Na]+ | 125.2*/303.1 | 14/30 | 35 |
| HT-2-3G | C28H42O13 | 586.2 | 604.3 [M + NH4]+ | 263.1*/245.1 | 12/13 | 20 |
| T-2-3G | C30H44O14 | 628.7 | 629.3 [M + H]+ | 305.1*/215.1 | 14/18 | 32 |
| HT-2 | C22H32O8 | 424.5 | 442.4 [M + NH4]+ | 215.2*/263.2 | 11/10 | 20 |
| T-2 | C24H34O9 | 466.5 | 484.4 [M + NH4]+ | 185.1*/305.2 | 18/14 | 32 |
Primary product ion
2.5. Method validation
Method validation was performed according to the European Commission decision 2002/657/EC, EMEA, and FDA guidelines. Linearity was evaluated by calculating the regression coefficient (R2), which was constructed by fortification in the neat solvent and cereal blank matrix at the concentration of 0.5–200 μg/L for T-2, HT-2, T-2-tetraol, and 0.5–100 μg/L for NEO, T-2-triol, T-2-3G, HT-2-3G. Limit of quantification (LOQ) and limit of detection (LOD) were designated as the concentration with a signal-to-noise (S/N) ratio of 3 and 10, respectively. Recoveries were also evaluated by spiking in blank samples at low, medium, and high concentration levels, each in six replicates. Simultaneously, the intra-day precision (repeatability) and inter-day precision (reproducibility) were estimated using the relative standard deviation (RSD) of different spiked levels on the same day and in five successive days, respectively. Signal suppression/enhancement (SSE), calculated as Eq. (1), was applied to assess the matrix effect (Azaiez, Giusti, Sagratini, Maes, & Fernández-Franzón, 2014).
| (1) |
2.6. Dietary exposure assessment
The dietary exposure (ng/kg b.w./day) to T-2 and its modified forms was calculated by multiplying their concentrations (ng/kg) by the cereal-based products consumption data (kg/kg b.w./day). To accurately assess dietary risk to the consumer, lower bound (LB) and upper bound (UB) scenarios of the concentration were considered, and the results below LOQ were replaced by zero or LOQ. As well, three scenarios (mean, maximum, P95) of the consumption data were included. The food consumption data of Shanghai inhabitants in different cereals and cereal-based products were obtained from the previous food consumption survey performed using face-to-face questionnaires (Dong et al., 2017, Yang et al., 2020, Yang et al., 2020).
3. Results and discussion
3.1. UPLC-MS/MS method establishment and validation
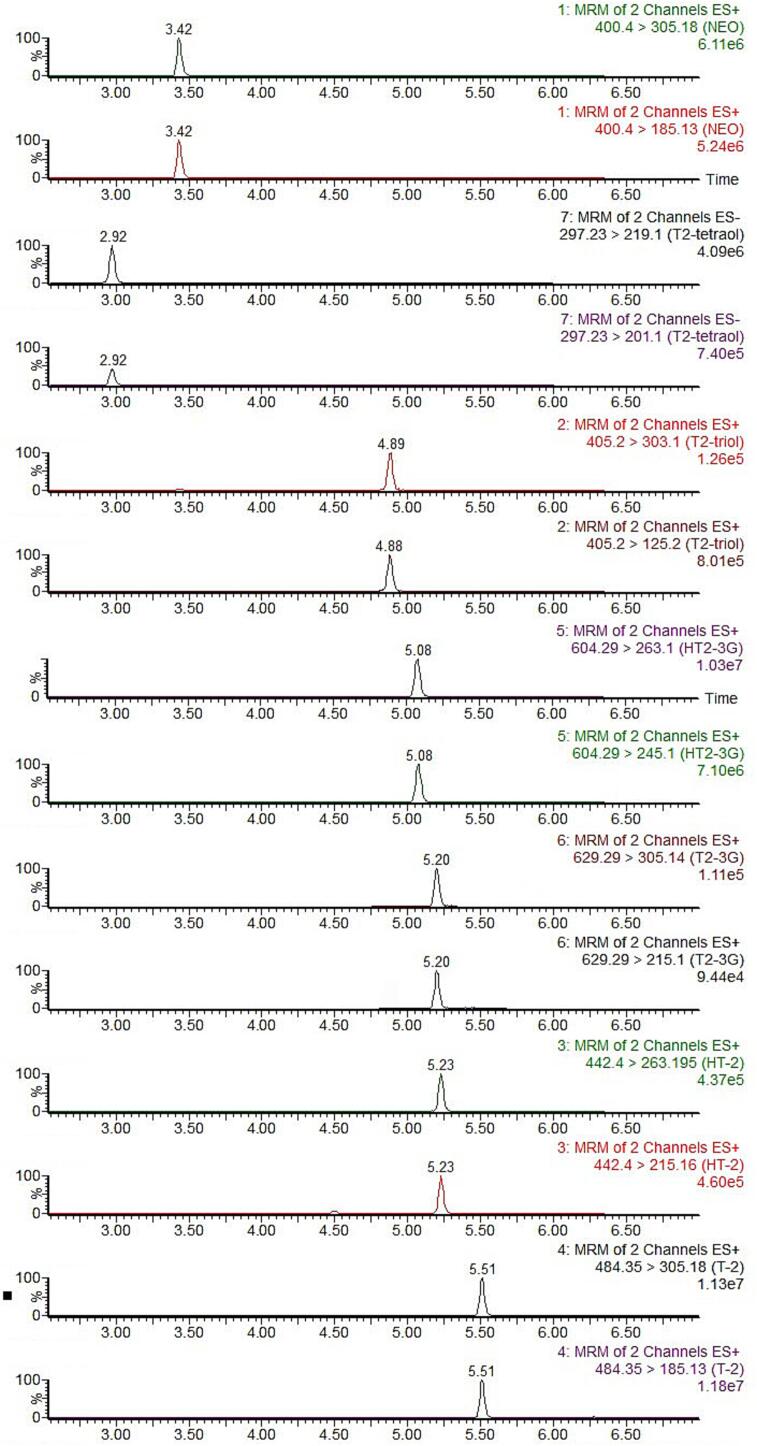
Satisfactory chromatographic separation, symmetrical peak shapes, and high signal intensities for T-2 and its modified forms, as shown in Fig. 2, were obtained after comprehensive optimization of chromatographic columns, mobile phase, and the MS parameters. Considering the complex matrices of cereals and cereal-based products, adequate sample pretreatment, including extraction and clean-up, is essential. As shown in Figure S1, using non-contaminated cereal samples spiked with 50 µg/kg of the targeted T-2 and its modified forms, satisfactory extraction recoveries (80.4–112.0 %) and purification recoveries (82.3–111.3 %) were acquired for the targeted T-2 and its modified forms when acetonitrile/water (84/16, v/v) was selected as the extraction solvent and HLB cartridges were used for the clean-up step.
Fig. 2.
Chromatograms of T-2 and its modified forms in methanol/water solution (20/80, v/v) at the concentration of 50 ng/mL for T-2, HT-2, NEO, T-2-triol, and T-2-3G, 200 ng/mL for T-2-tetraol and HT-2-3G.
As shown in Table S2, all the correlation coefficients (R2) of the calibration curve of T-2 and its modified forms were ≥ 0.99. The LOD and LOQ for T-2 and its modified forms in different matrices were in the range of 0.2–5 μg/kg and 0.5–10 μg/kg, respectively. The recoveries of T-2 and its modified forms ranged from 76.8 to 115.2 % in maize and maize flour, 86.9–110.4 % in wheat, 84.3–106.0 % in wheat flour, 78.8–108.9 % in rice, 78.8–108.9 % in sorghum, 83.2–104.4 % in barley, and 84.6–110.4 % in oatmeal. The intra- and inter-day RSDs were in the range of 0.9–12.8 % and 3.5–15.8 %, respectively. SSE values varied from 9.3 % to 38.7 % in maize and maize flour, 7.6 % to 21.7 % in wheat, 3.4 % to 24.2 % in wheat flour, 1.2 % to 9.8 % in rice, 8.3 % to 28.0 % in sorghum, 5.6 % to 20.7 % in barley, and 2.3 % to 27.6 % in oatmeal. These results indicated that the matrix effect remained higher, especially for T-2, T-2-tetraol, and HT-2-3G in maize and maize flour even though a HLB cleanup step had been applied. Consequently, matrix-matched calibration curves were used for accurate quantification of all targeted analytes. Compared with previous studies (Table S3), the performances of the recovery, sensitivity, and matrix effect in current study are similar or even better, and the pretreatment method is much cheaper and simpler than clean-up by immunoaffinity column, Mycosep® 227 column, and Bond Elut Mycotoxin® column (Bernhardt et al., 2016, Marianna et al., 2006, Tahoun et al., 2021). As shown in Table S4, the comparison results between this method and those methods based on LC-FLD (National Standard of the People's Republic of China), LC-MS/MS (Lu, Guo, Tian, Luo, & Yang, 2022), and LC-HRMS (Lattanzio, Ciasca, Terzi, Ghizzoni, McCormick, & Pascale, 2015) in previous reports suggested that the proposed method was accurate, sensitive, and reproducible, and that it could be used for the accurate detection of T-2 and its modified forms in cereals and cereal-based products.
3.2. Screening of commercial samples
A total of 107 cereals and cereal-based products were analyzed for the occurrence of T-2, HT-2, NEO, T-2-triol, T-2-tetraol, T-2-3G, and HT-2-3G. The incidences (%), concentration range, as well as the median values of the mycotoxins found in each type of cereal were presented in Table 2. Incidences (%) were calculated according to the number of samples with concentrations > LOD divided by the total number of samples.
Table 2.
Occurrence and concentrations (μg/kg) of T-2 and its modified forms in different cereals and cereal-based products.
| Sample | T-2 | HT-2 | NEO | T-2-triol | T-2-tetraol | T-2-3G | HT-2-3G |
|---|---|---|---|---|---|---|---|
| Maize (n = 20) | |||||||
| %posa | 30 | 25 | 10 | 5 | 25 | 10 | 15 |
| Meanb (μg/kg) | 12.73 | 16.89 | 6.29 | 2.11 | 4.72 | 0.88 | 1.66 |
| Rangec (μg/kg) | 0.51–56.61 | 0.92–45.43 | 0.22–12.35 | 2.11 | 1.12–10.03 | 0.39–1.36 | 0.55–3.31 |
| Maize flour (n = 11) | |||||||
| %pos | 27.3 | 18.2 | n.d.d | n.d. | 9.1 | n.d. | 9.1 |
| Mean (μg/kg) | 2.20 | 4.55 | n.d. | n.d. | 0.87 | n.d. | 0.54 |
| Range (μg/kg) | 0.94–3.11 | 1.26–7.84 | n.d. | n.d. | 0.87 | n.d. | 0.54 |
| Wheat (n = 12) | |||||||
| %pos | 16.7 | 25 | n.d. | n.d. | 16.7 | 8.3 | n.d. |
| Mean (μg/kg) | 14.05 | 9.84 | n.d. | n.d. | 3.91 | 1.13 | n.d. |
| Range (μg/kg) | 2.22–25.88 | 1.67–22.1 | n.d. | n.d. | 1.02–6.79 | 1.13 | n.d. |
| Wheat flour (n = 20) | |||||||
| %pos | 10 | 10 | 5 | n.d. | n.d. | 10 | 10 |
| Mean (μg/kg) | 6.88 | 1.13 | 2.11 | n.d. | 2.06 | n.d. | 2.65 |
| Range (μg/kg) | 2.19–11.56 | 0.87–1.38 | 2.11 | n.d. | 0.91–3.21 | n.d. | 0.58–4.72 |
| Sorghum (n = 12) | |||||||
| %pos | 25 | 16.7 | 8.3 | 16.7 | n.d. | n.d. | 8.3 |
| Mean (μg/kg) | 8.87 | 4.69 | 3.65 | 4.45 | n.d. | n.d. | 0.66 |
| Range (μg/kg) | 1.28–14.57 | 2.49–6.88 | 3.65 | 3.13–5.77 | n.d. | n.d. | 0.66 |
| Rice (n = 20) | |||||||
| %pos | 10 | 10 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Mean (μg/kg) | 1.05 | 0.64 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Range (μg/kg) | 0.47–1.63 | 0.36–0.92 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Barley (n = 4) | |||||||
| %pos | 75 | 50 | n.d. | n.d. | 50 | 25 | 25 |
| Mean (μg/kg) | 28.18 | 62.68 | n.d. | n.d. | 7.88 | 1.13 | 2.31 |
| Range (μg/kg) | 1.27–78.51 | 10.22–115.13 | n.d. | n.d. | 3.65–12.1 | 1.13 | 2.31 |
| Oatmeal (n = 8) | |||||||
| %pos | 12.5 | 12.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Mean (μg/kg) | 0.68 | 1.33 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Range (μg/kg) | 0.68 | 1.33 | n.d. | n.d. | n.d. | n.d. | n.d. |
Percentage of samples with a concentration above the limit of detection (LOD).
Mean concentration of the positive samples (μg/kg).
Concentration range of the positive samples (μg/kg).
n.d. means T-2 and its modified forms were not detected (<LOD).
As seen in Table 2, one or multiple of T-2 and its modified forms were simultaneously detected in different cereals and cereal-based products. In maize and maize flour, T-2 and HT-2 occurred in higher incidences, and were found in 29.0 % (9/31) and 22.6 % (7/31) of samples with the concentrations ranging 0.51–56.61 μg/kg and 0.92–45.43 μg/kg, respectively. The modified forms including NEO, T-2-triol, T-2-tetraol, T-2-3G and HT-2-3G were detected separately or simultaneously in 32.3 % (10/31) of all maize and maize flour samples; their concentrations were in ranges from 0.22 to 12.35 μg/kg for NEO, 2.11 μg/kg for T-2-triol, 0.87 to 10.03 μg/kg for T-2-tetraol, 0.39 to 1.36 μg/kg for T-2-3G, and 0.54 to 3.31 μg/kg for HT-2-3G. The concentrations of T-2 and its modified forms determined in maize and maize flour were generally lower or equal to those reported in previous studies (Lattanzio et al., 2015, Meng-Reiterer et al., 2015, Schmidt et al., 2018) and the maximum concentrations detected were also lower than the regulative limits set for the sum of T-2/HT-2 (200 μg/kg) in maize-based products.
In wheat and wheat flour, the incidence of positive samples for T-2 and its modified forms was 27.3 % with concentrations ranging from 2.19 to 25.88 μg/kg for T-2, 0.87 to 22.10 μg/kg for HT-2, 2.11 μg/kg for NEO, 0.91–6.79 μg/kg for T-2-tetraol, 1.13 μg/kg for T-2-3G, and 0.58 to 4.72 μg/kg for HT-2-3G. The incidence and concentrations of T-2 and HT-2 were lower than those reported by Kassim et al. in wheat/wheat powder samples from the South Korea traditional market (T2: 65.2–431.0 μg/kg; HT-2: 30.8–355.3 μg/kg) (Kassim, Kim, Mtenga, Song, & Chung, 2011).
In rice and oatmeal, only T-2 and HT-2 were detected in 15 % (3/20) and 25 % (2/8) of the analyzed samples. The obtained concentrations ranged from 0.47 to 1.63 μg/kg for T-2 and 0.36 to 0.92 μg/kg for HT-2 in rice, and ranged from 0.68 μg/kg for T-2 and 1.33 μg/kg for HT-2 in oatmeal, all which were lower than concentrations found in previous reports. In sorghum, 33.3 % (4/12) of samples were positive with concentrations ranging from 1.28 to 14.57 μg/kg for T-2, 2.49 to 6.88 μg/kg for HT-2, 3.65 μg/kg for NEO, 3.17–5.77 for T-2-triol, and one sample positive for HT-2-3G with a concentration of 0.66 μg/kg. As one of the grains that are most susceptible to T-2 contamination based on results from the EU, 75.0 % (3/4) of barley samples were positive, with concentrations in ranges from 1.27 to 78.51 μg/kg for T-2, 10.22 to 115.13 μg/kg for HT-2, 3.65 to 12.1 μg/kg for T-2-tetraol, 1.13 for T-2-3G, and 2.31 μg/kg for HT-2-3G. Compared to previous reports on barley samples from Finland (Nathanail et al., 2015, Nathanail et al., 2015) and Northern Italy (Lattanzio et al., 2015), barley samples marketed in Shanghai is potentially contaminated by T-2 and its modified forms, but at lower levels, especially for HT-2-3G.
3.3. Co-occurrence of T-2 and its modified forms
It has been demonstrated that the T-2 and HT-2 toxins generally occur together in cereals and cereal-based products. However, studies on the co-occurrence of other modified forms of T-2 in cereals or cereal-based products are lacking. Fig. 3 presents the results of the co-occurrence of T-2 and its modified forms in the analyzed cereal samples.
Fig. 3.
Co-occurrence of T-2 and its modified forms in the contaminated cereals and cereal-based products. Ⅰ, Ⅱ, Ⅲ, and Ⅴ represents the number of contaminated T-2 forms.
Among the positive samples, 66.7 % were contaminated with two or more T-2 forms, and the remaining 33.3 % were contaminated by only one T-2, HT-2, or T-2-tetraol. In samples contaminated with two or more T-2 forms, thirteen different combinations of two to five mycotoxins were documented. Within these samples, the combination of T-2 and HT-2 was the most common with an incidence of 50 %. The highest number of T-2 forms occurring simultaneously (five) was found in two samples, one maize (T-2, HT-2, T-2-triol, T-2-tetraol, HT-2-3G) and one barley (T-2, HT-2, T-2-tetraol, T-2-3G, HT-2-3G).
Among different cereals categories analyzed in this study, sorghum was the most susceptible to contamination by T-2 and its modified forms, and 100.0 % of the positive samples were contaminated by two or more T-2 forms. Similarly, maize and maize flour (76.9 %), barley (75.0 %), as well as wheat and wheat flour (66.7 %) were also susceptible to contamination by various T-2 forms. Interestingly, in previous reports by Broekaert et al., the total number of samples containing T-2-3G (73 %) and HT-2-3G (80 %) was higher than samples contaminated by T-2 and HT-2 despite substantially lower concentrations of the former two mycotoxins (Broekaert, Devreese, Baere, Backer, & Croubels, 2015). Moreover, NEO, T-2-tetrol, and T2-tetraol were also found along with T-2 and HT-2 in very few available literatures (Nakagawa et al., 2013, Pernica et al., 2022). These results clearly indicate that the modified forms of T-2 and HT-2 often coexist with their parent compounds despite infrequent reports.
3.4. Dietary exposure assessment
To obtain a more accurate estimation of intake and the risk to adults from T-2 and its modified forms through cereals and cereal-based products, a probabilistic dietary exposure assessment was used. All cereal samples were divided into four categories (rice and rice products, wheat and wheat products, maize and maize products, and other cereals and their products). The results of dietary exposure to the sum of T-2 forms (minimum, mean, maximum, P50, P75, P95) (ng/kg b.w./day) in the adult population of Shanghai within LB and UB concentration scenarios are presented in Table 3.
Table 3.
Dietary exposure to the sum T-2 and its modified forms (ng/kg b.w./day) in Shanghai adults from different cereals and cereal-based products using probabilistic exposure assessment.
| Dietary exposure of sum T-2 eq (ng/kg b.w./day) | Rice | Wheat and wheat flour | Maize and maize flour | Other cereals and their products | ||||
|---|---|---|---|---|---|---|---|---|
| LBa | UBb | LB | UB | LB | UB | LB | UB | |
| Min | 0.00 | 0.00 | 0.00 | 0.29 | 0.00 | 0.64 | 0.00 | 0.00 |
| Mean | 0.02 | 0.03 | 0.57 | 0.96 | 0.07 | 3.63 | 0.06 | 0.25 |
| P5 | 0.00 | 0.00 | 0.03 | 0.32 | 0.00 | 0.87 | 0.00 | 0.02 |
| P25 | 0.00 | 0.00 | 0.16 | 0.48 | 0.02 | 1.84 | 0.00 | 0.06 |
| P50 | 0.02 | 0.03 | 0.39 | 0.75 | 0.05 | 3.26 | 0.04 | 0.13 |
| P75 | 0.07 | 0.09 | 0.79 | 1.22 | 0.10 | 5.12 | 0.13 | 0.28 |
| P95 | 0.16 | 0.17 | 1.70 | 2.31 | 0.21 | 7.59 | 0.31 | 0.89 |
| Max | 0.35 | 0.35 | 5.39 | 6.55 | 0.66 | 9.57 | 1.10 | 5.60 |
LB: lower bound (analytical data < LOQ = 0); bUB: upper bound (analytical data < LOQ = LOQ).
The mean dietary exposure values of the sum of the various T-2 forms in four cereals categories ranged from 0.02 to 0.57 ng/kg b.w./day for LB, 0.03 to 3.63 ng/kg b.w./day for UB. Similarly, the P95 values ranged from 0.16 to 1.70 ng/kg b.w./day for LB, and 0.17 to 7.59 ng/kg b.w./day for UB. All probabilistic values along with the maximum dietary exposure values were lower than the tolerable daily intake (TTC) value of 20 ng/kg b.w./day established by the CONTAM panel of EFSA in 2017 (EFSA, 2017). However, the mean, P75, P95 as well as the maximum dietary exposure (UB) for the sum of T-2 forms through maize and maize flour were 18.15 %, 25.60 %, 7.59 %, and 47.85 % of the TDI, respectively, suggesting that the concentrations of T-2 and its modified forms in maize and maize flour could cause some degree of health risk to the adult population in Shanghai.
The mean and P95 probabilistic exposure values in this study were consistent with results in the dietary exposure assessment on Czech adult consumers using the sum of T-2/HT-2 from cereal-based foods (n = 336), in which the mean and P95 exposure values ranged from 0.5 to 2.9 ng/kg b.w./day (LB-UB), and 1.9 to 11.5 ng/kg b.w./day, respectively (Ostry et al., 2020). On the other hand, the mean and P95 daily exposures to all T-2 forms in adults via cereal-based foods were far lower than those estimated for Belgian habitants (De Boevre et al., 2013). As well, Stanciu et al. analyzed T2 and HT2 in wheat-based products consumed by the Romanian adult population and found that dietary intake for T-2 and HT-2 was 65 ng/kg b.w./day (UB) (Stanciu, Juan, Miere, Berrada, Loghin, & Mañes, 2018). In addition, the results from the Second French Total Diet Study showed that mean and P95 exposure to the sum of T2/HT2 in adults ranged from 9 to 52 ng/kg b.w./day (LB-UB) and from 19 to 95 ng/kg b.w./day, respectively (Sirot, Fremy, & Leblanc, 2013). The differences found in dietary exposures to all T-2 forms are determined by a combination of the type and quantity of consumed cereal, the concentrations of T-2 and its various forms, the detection methods utilized, as well as the food consumption data.
4. Conclusion
In this study, a reliable and sensitive UPLC-MS/MS method was established and applied in screening for seven T-2 forms in commercially available cereals and cereal-based products in Shanghai. Of the 107 analyzed samples, 30.8 % were found to be contaminated by at least one form of T-2. Importantly, 66.7 % of the positive samples were simultaneously contaminated with two or more T-2 forms, many of which have often been overlooked in previous studies. This study also assessed the risk associated with exposure to T-2 and its modified forms through intake of cereal and cereal-based products. To the best of our knowledge, it is the first study on dietary exposure to T-2 that includes its multiple modified forms. Although dietary exposure values of the sum of the T-2 forms did not exceed the TDI of the sum of T-2 and HT-2, our results demonstrate that a systematic and comprehensive monitoring of T-2 forms remains necessary to effectively protect human health due to their toxicity and potential conversion into T-2.
CRediT authorship contribution statement
Wenbo Guo: Methodology, Writing – original draft. Disen Feng: Investigation. Xianli Yang: Software, Visualization. Zhihui Zhao: Writing – review & editing. Junhua Yang: Conceptualization, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Shanghai Rising-Star Program (21QB1403300), and Science and Technology Commission of Shanghai Municipality (21DZ1201300).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101199.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Azaiez I., Giusti F., Sagratini G., Maes J., Fernández-Franzón M. Multi-mycotoxins analysis in dried fruit by LC/MS/MS and a modified QuEChERS procedure. Food Analytical Methods. 2014;7(4):935–945. [Google Scholar]
- Bernhardt K., Valenta H., Kersten S., Humpf H.U., Dnicke S. Determination of T-2 toxin, HT-2 toxin, and three other type a trichothecenes in layer feed by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS)–COMPARISON of two sample preparation methods. Mycotoxin Research. 2016;32(2):89–97. doi: 10.1007/s12550-016-0244-z. [DOI] [PubMed] [Google Scholar]
- Broekaert N., Devreese M., Baere S.D., Backer P.D., Croubels S. Modified fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food and Chemical Toxicology. 2015;80:17–31. doi: 10.1016/j.fct.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Chen P., Xiang B., Shi H., Yu P., Song Y., Li S. Recent advances on type a trichothecenes in food and feed: Analysis, prevalence, toxicity, and decontamination techniques - ScienceDirect. Food Control. 2020;118 [Google Scholar]
- Commission Recommendation Commission recommendation (EU) on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off J Eur Union. 2013;L91:12–15. [Google Scholar]
- De Boevre M., Jacxsens L., Lachat C., Eeckhout M., Di Mavungu J.D., Kris A., De Saeger S. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicology Letters. 2013;218(3):281–292. doi: 10.1016/j.toxlet.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Dong R., Zhou T., Zhao S., Zhang H., Zhang M., Chen J., Chen B. Food consumption survey of Shanghai adults in 2012 and its associations with phthalate metabolites in urine. Environment international. 2017;101:80–88. doi: 10.1016/j.envint.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Drakopoulos D., Sulyok M., Krska R., Logrieco A.F., Vogelgsang S. Raised concerns about the safety of barley grains and straw: A swiss survey reveals a high diversity of mycotoxins and other fungal metabolites. Food. 2021;Control(7) [Google Scholar]
- Efsa Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA Journal. 2011;9(12):2481–2668. [Google Scholar]
- Efsa Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA Journal. 2017;15(1):4655–4708. doi: 10.2903/j.efsa.2017.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gab-Allah M.A., Tahoun I.F., Yamani R.N., Rend E.A., Shehata A.B. Eco-friendly and sensitive analytical method for determination of T-2 toxin and HT-2 toxin in cereal products using UPLC-MS/MS. Journal of Food Composition and Analysis. 2022;107 [Google Scholar]
- Kasimir M., Behrens M., Schulz M., Kuchenbuch H., Focke C., Humpf H.-U. Intestinal metabolism of α- and β-glucosylated modified mycotoxins T-2 and HT-2 toxin in the pig cecum model. Journal of agricultural and food chemistry. 2020;68(19):5455–5461. doi: 10.1021/acs.jafc.0c00576. [DOI] [PubMed] [Google Scholar]
- Kassim N., Kim K., Mtenga A.B., Song J.E., Chung D.H. A preliminary study of T-2 and HT-2 toxins in cereals sold in traditional market in South Korea. Food Control. 2011;22(8):1408–1412. [Google Scholar]
- Kiš M., Vuli A., Kudumija N., Arkanj B., Pleadin J. A two-year occurrence of fusarium T-2 and HT-2 toxin in croatian cereals relative of the regional weather. Toxins. 2021;13(1):39. doi: 10.3390/toxins13010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochiieru Y., Mankevičienė A., Cesevičienė J., Semaškienė R., Dabkevičius Z., Janavičienė S. The influence of harvesting time and meteorological conditions on the occurrence of fusarium species and mycotoxin contamination of spring cereals. Journal of the Science of Food and Agriculture. 2020;100(7):2999–3006. doi: 10.1002/jsfa.10330. [DOI] [PubMed] [Google Scholar]
- Lattanzio V.M.T., Ciasca B., Terzi V., Ghizzoni R., McCormick S.P., Pascale M. Study of the natural occurrence of T-2 and HT-2 toxins and their glucosyl derivatives from field barley to malt by high-resolution orbitrap mass spectrometry. Food Additives & Contaminants. 2015;32(10):1647–1655. doi: 10.1080/19440049.2015.1048750. [DOI] [PubMed] [Google Scholar]
- Ling A., Sun L., Guo W., Sun S., Yang J., Zhao Z. Individual and combined cytotoxic effects of T-2 toxin and its four metabolites on porcine Leydig cells Food and Chemical. 2020;Toxicology(139) doi: 10.1016/j.fct.2020.111277. [DOI] [PubMed] [Google Scholar]
- Lu Q., Guo M.Y., Tian J., Luo J.Y., Yang M.H. A comprehensive study on multi-mycotoxin screening, changes of mycotoxin residues and fungal community analysis from barley germination to malt. International Journal of Food Microbiology. 2022;372 doi: 10.1016/j.ijfoodmicro.2022.109678. [DOI] [PubMed] [Google Scholar]
- Mankevičienė A., Gaurilčikienė I., Dabkevičius Z., Semaškienė R., Mačkinaitė R., Supronienė S. Mycotoxin contamination of lithuanian-grown cereal grains and factors determining it mycotoxin contamination of lithuanian-grown cereal grains and factors determining it. Ekologija. 2006;3:21–27. [Google Scholar]
- Marianna K., Lauber U., Humpf H.-U. A new solid phase extraction clean-up method for the determination of 12 type a and B trichothecenes in cereals and cereal-based food by LC-MS/MS. Molecular Nutrition & Food Research. 2006;50(3):261–269. doi: 10.1002/mnfr.200500234. [DOI] [PubMed] [Google Scholar]
- McCormick S.P., Kato T., Maragos C.M., Busman M., Lattanzio V.M.T., Galaverna G., Kurtzman C.P. Anomericity of T-2 toxin-glucoside: Masked mycotoxin in cereal crops. Journal of agricultural and food. 2015;chemistry(63):731–738. doi: 10.1021/jf504737f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S.P., Price N.P.J., Kurtzman C.P. Glucosylation and other biotransformations of T-2 toxin by yeasts of the trichomonascus clade. Applied & Environmental Microbiology. 2012;78(24):8694. doi: 10.1128/AEM.02391-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng-Reiterer J., Varga E., Nathanail A.V., Bueschl C., Rechthaler J., McCormick S.P., Schuhmacher R. Tracing the metabolism of HT-2 toxin and T-2 toxin in barley by isotope-assisted untargeted screening and quantitative LC-HRMS analysis. Analytical and Bioanalytical Chemistry. 2015;407(26):8019–8033. doi: 10.1007/s00216-015-8975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Sakamoto S., Sago Y., Kushiro M., Nagashima H. Detection of masked mycotoxins derived from type a trichothecenes in corn by high-resolution LC-orbitrap mass spectrometer. Food Additives & Contaminants: Part A. 2013;30(8):1407–1414. doi: 10.1080/19440049.2013.790087. [DOI] [PubMed] [Google Scholar]
- Nathanail A.V., Syvähuoko J., Malachová A., Jestoi M., Varga E., Michlmayr H., Peltonen K. Simultaneous determination of major type a and B trichothecenes, zearalenone and certain modified metabolites in finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Analytical and Bioanalytical Chemistry. 2015;407:4745–4755. doi: 10.1007/s00216-015-8676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanail A.V., Varga E., Meng-Reiterer J., Bueschl C., Michlmayr H., Malachova A., Berthiller F. Metabolism of the fusarium mycotoxins T-2 toxin and HT-2 toxin in wheat. Journal of Agricultural & Food Chemistry. 2015;63(35):7862–7872. doi: 10.1021/acs.jafc.5b02697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Standard of the People's Republic of China. Determination of T-2 toxin in food: GB 5009.118-2016, Beijing, China.
- Ostry V., Dofkova M., Blahova J., Malir F., Kavrik R., Rehurkova I., Ruprich J. Dietary exposure assessment of sum deoxynivalenol forms, sum T-2/HT-2 toxins and zearalenone from cereal-based foods and beer. Food and Chemical Toxicology. 2020;139 doi: 10.1016/j.fct.2020.111280. [DOI] [PubMed] [Google Scholar]
- Ostry V., Malir F., Toman J., Grosse Y. Mycotoxins as human carcinogens—the IARC monographs classification. Mycotoxin Research. 2017;33:65–73. doi: 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- Pereira V.L., Fernandes J.O., Cunha S.C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type a and type B) in processed cereal-based baby foods by GC-MS. Food Chemistry. 2015;182:143–149. doi: 10.1016/j.foodchem.2015.01.047. [DOI] [PubMed] [Google Scholar]
- Pernica, M., Blanka.Kyralová, Zdeněk.Svoboda, Boko, R., Iveta.Broková, Lenka.eslová, . . . Sylvie.Běláková. (2022). Levels of T-2 toxin and its metabolites, and the occurrence of Fusarium fungi in spring barley in the Czech Republic. Food microbiology, 102, 103875. [DOI] [PubMed]
- Pleadin, J., Vasilj, V., Kudumija, N., Petrović, D., Vilušić, M., & Škrivanko, M. (2017). Survey of T-2/HT-2 toxins in unprocessed cereals, food and feed coming from Croatia and Bosnia & Herzegovina. Food Chemistry, 224, 153-159. [DOI] [PubMed]
- Schmidt H.S., Schulz M., Focke C., Becker S., Cramer B., Humpf H.U. Glucosylation of T-2 and HT-2 toxins using biotransformation and chemical synthesis: Preparation, stereochemistry, and stability. Mycotoxin. 2018;Research(34):159–172. doi: 10.1007/s12550-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot V., Fremy J.-M., Leblanc J.C. Dietary exposure to mycotoxins and health risk assessment in the second french total diet study. Food & Chemical Toxicology. 2013;52:1–11. doi: 10.1016/j.fct.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Stanciu O., Juan C., Miere D., Berrada H., Loghin F., Mañes J. First study on trichothecene and zearalenone exposure of the romanian population through wheat-based products consumption. Food and Chemical Toxicology. 2018;121:336–342. doi: 10.1016/j.fct.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Tahoun I.F., Gab-Allah M.A., Yamani R.N., Shehata A.B. Development and validation of a reliable LC-MS/MS method for simultaneous determination of deoxynivalenol and T-2 toxin in maize and oats. Microchemical Journal. 2021;169(9) [Google Scholar]
- Taroncher M., Rodríguez Carrasco Y., Ruiz M.J. Interactions between T-2 toxin and its metabolites in HepG2 cells and in silico approach. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research. 2021;148 doi: 10.1016/j.fct.2020.111942. [DOI] [PubMed] [Google Scholar]
- Wu Q., Qin Z., Kuca K., You L., Zhao Y., Liu A., Wang X. An update on T-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Archives of Toxicology. 2020;94:3645–3669. doi: 10.1007/s00204-020-02899-9. [DOI] [PubMed] [Google Scholar]
- Yang J., Guo W., Wang J., Yang X., Zhang Z., Zhao Z. T-2 toxin-induced oxidative stress leads to imbalance of mitochondrial fission and fusion to activate cellular apoptosis in the human liver 7702 cell line. Toxins. 2020;12:43. doi: 10.3390/toxins12010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Poucke C.V., Wang Z., Zhang S., Saeger S.D., Boevre M.D. Metabolic profile of the masked mycotoxin T-2 toxin-3-glucoside in rats (in vitro and in vivo) and humans (in vitro) World Mycotoxin Journal. 2017;10(4):349–362. [Google Scholar]
- Yang X., Zhao Z., Tan Y., Chen B., Wu A. Risk profiling of exposures to multiclass contaminants through cereals and cereal-based products consumption: A case study for the inhabitants in Shanghai. China. Food Control. 2020;109 [Google Scholar]
- Zhang X., Wang Y., Yang X., Liu M., Li Y. The nephrotoxicity of T-2 toxin in mice caused by oxidative stress-mediated apoptosis is related to Nrf2 pathway. Food and Chemical Toxicology. 2021;149 doi: 10.1016/j.fct.2021.112027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.