Fig. 5.
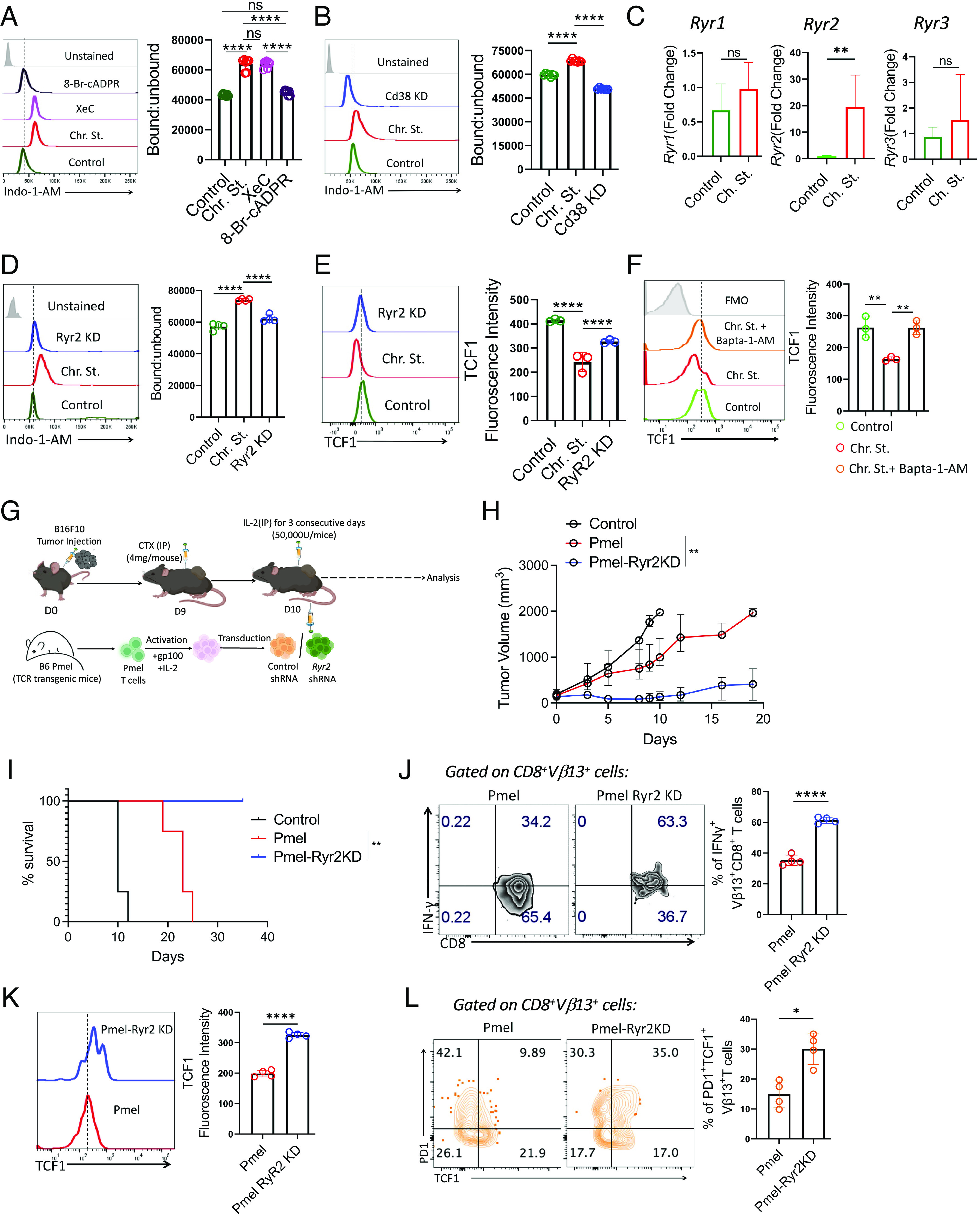
CD38 induces elevation of intracellular Ca2+ levels through the cADPR–RyR2 axis. (A and B) Intracellular calcium level as measured by ratio of bound to unbound Indo-1-AM in A control and chronically stimulated T cells treated either with XeC (5 µM) or 8-Br-cADPR (2.5 µM), (B) control and chronically stimulated T cells transduced either with control shRNA or CD38 shRNA. (C) Transcript levels of Ryr1, Ryr2, and Ryr3. (D and E) Control and chronically stimulated T cells transduced either with control shRNA or Ryr2 shRNA were assessed for (D) intracellular calcium level as measured by the ratio of bound to unbound Indo-1-AM and (E) expression of TCF1. (F) Control and chronic stimulated CD8+ T cells treated with or without Bapta-1-AM (13 µM) were evaluated for the expression of TCF1. (G) Schematic presentation of the experimental strategy and the differences observed in (H) tumor growth and (I) survival of tumor-bearing mice when subcutaneously established B16-F10 tumor in C57BL/6 mice (n = 4 mice/group) were treated by adoptively transferring 0.75 × 106 Pmel T cells transduced with either control shRNA or Ryr2 shRNA. (J and K) Vβ13+CD8+ T cells retrieved from the tumor site were evaluated for (J) intracellular production of IFNγ, and (K) expression of TCF1. (L) Frequencies of tumor-derived Vβ13+CD8+ T cells expressing PD1 and TCF1. Adjacent bars represent cumulative data from (A and B) seven, (C and D) four, (E and F) cumulative data of mean fluorescence intensity from three, (L) frequency of PD1+TCF1+ T cells from four independent experiments. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001; ns, nonsignificant.
