Significance
This study non-invasively investigates blood flow in different regions of the hippocampus using advanced MR imaging techniques in clinically feasible times. The researchers found significant variations in perfusion among subfields of the hippocampus, with one subfield, CA1, showing the lowest perfusion levels. The differences in perfusion were detected quickly and reliably. The study assessed various contributing factors to these variations, including image quality, tissue properties, blood vessels, and neuronal density. The findings suggest that proximity to arteries and the density of neurons play important roles in hippocampal perfusion. Understanding these fine-grained differences in perfusion enhances our knowledge of hippocampal function and its role in healthy aging and in neurological disorders.
Keywords: hippocampus, subfields, perfusion, vasculature, 7 tesla MRI
Abstract
We present a comprehensive study on the non-invasive measurement of hippocampal perfusion. Using high-resolution 7 tesla arterial spin labeling (ASL) data, we generated robust perfusion maps and observed significant variations in perfusion among hippocampal subfields, with CA1 exhibiting the lowest perfusion levels. Notably, these perfusion differences were robust and already detectable with 50 perfusion-weighted images per subject, acquired in 5 min. To understand the underlying factors, we examined the influence of image quality metrics, various tissue microstructure and morphometric properties, macrovasculature, and cytoarchitecture. We observed higher perfusion in regions located closer to arteries, demonstrating the influence of vascular proximity on hippocampal perfusion. Moreover, ex vivo cytoarchitectonic features based on neuronal density differences appeared to correlate stronger with hippocampal perfusion than morphometric measures like gray matter thickness. These findings emphasize the interplay between microvasculature, macrovasculature, and metabolic demand in shaping hippocampal perfusion. Our study expands the current understanding of hippocampal physiology and its relevance to neurological disorders. By providing in vivo evidence of perfusion differences between hippocampal subfields, our findings have implications for diagnosis and potential therapeutic interventions. In conclusion, our study provides a valuable resource for extensively characterizing hippocampal perfusion.
The brain’s multiscale organization enables processing of different sensory inputs through pathways optimized for storing, updating, and recollecting relevant information (1). In particular, the structure and function of the hippocampus (or “hippocampal formation”) have been at the center of attention in a plethora of studies focused on the brain and cognitive aging, especially those investigating memory (dys)function, where it was found to be involved in episodic memory (i.e., encoding and retrieval of information tied to a specific time and place), as well as in other types of declarative memory (2).
Although the hippocampus has been studied as a singular region for several years, emerging in vivo imaging (e.g., ultra-high field MRI) and analysis (e.g., topologically correct unfolding) methods have enabled a better appreciation of its internal organization (3, 4). While a lot is known about the histological subdivisions of the hippocampal formation (5), several studies have provided in vivo evidence of hippocampal subfields namely, the subiculum (Sub), the Cornu Ammonis (CA) fields 1 to 4 and Dentate Gyrus (DG), their unique anatomical properties (6, 7) and their distinct roles in memory processing (8, 9) and sensitivity to age-related changes (10–13). The fact that there are subfield-specific properties likely render differential effects across diseases (14) and disease subtypes, such as those observed in focal epilepsy (15). Unfortunately, the neurobiological substrates underlying age- or disease-related changes across and between subfields remain poorly understood. While it is known that hippocampal subfields differ in their molecular profiles (16–18), such as expression of NMDA and mineralocorticoid receptors, and the flexibility to deal with metabolic insults (e.g., hypoxia, ischemia, and reductions in the level of circulating hormones) (19, 20), these factors provide only partial substrates for the selective vulnerability. It is hypothesized that non-molecular factors play a role as well, such as differences in cytoarchitecture (3), vascular density (21) and physiology (e.g., spiking rate) (22). Regional differences in metabolic demand due to their unique composition of cell types, vascular density, and activity are likely to render hippocampal subfields differently perfused by blood. Recent efforts using high-resolution time-of-flight magnetic resonance angiography (TOF-MRA) data enabled differentiation of hippocampal vascularization patterns and assessment of their impact on cognitive functioning in cerebral small vessel disease patients (23, 24). Similarly, ferumoxytol-enhanced susceptibility-weighted imaging revealed differences across subfields in terms of microvascular density (25). Nonetheless, it has remained unclear how these macro- and microvascularization patterns translate to variability in the amount of blood (in mL/100 g/min) perfused in the hippocampal tissue. As such, in vivo characterization of subfield perfusion will provide important insight to further our understanding of the hippocampal functioning in health and in disease.
Arterial spin labeling (ASL) is a non-invasive MRI method that allows quantitative measurements of cerebral perfusion (26). ASL relies on arterial blood water as endogenous tracer, but typically suffers from low signal-to-noise ratio (SNR) due to low gray matter microvascular density relative to the tissue volume (27). At 3 tesla (3T), averaging tens of images acquired in roughly 2 to 4 min can provide a low-resolution (4 mm isotropic) perfusion map using ASL. However, this spatial resolution is insufficient to delineate perfusion differences across the hippocampus, which has a more fine-grained neuroanatomical composition. Hippocampal anatomy varies considerably between individuals (28) and its fine details are indistinguishable using standard anatomical T1-weighted scans. In most cases, specialized coronal T2-weighted scans with a high in-plane resolution positioned oblique to the hippocampus’s long axis are used to delineate its convoluted anatomy (29). Regardless, out-of-plane issues during manual, voxel-based labeling procedures render it difficult to respect topological constraints such as the contiguity of hippocampal subfields (4, 30). This fine-grained anatomical structure in addition to the inter-subject variability (28) limits the ability to perform simple across-subjects averaging to improve SNR of the ASL data.
In this study, we tackle the aforementioned challenges in characterizing hippocampal perfusion by capitalizing upon advances in acquisition and analysis strategies. For our first goal, we optimized an ASL acquisition scheme at 7T and leverage the gain in SNR with field strength and lengthening T1 at UHF to obtain robust high-resolution (1.5 mm3) hippocampal perfusion data (31, 32). The characterization of the hippocampal anatomy is facilitated using sub-millimeter resolution T2-weighted data and construction of a surface-based representation using the HippUnfold approach (33). By modeling the hippocampus as a folded surface, this approach circumvents issues experienced with manual voxel-based methods and enables inter-subject alignment, as well as parcellation based on a subfield atlas that respects topological constraints (4). Joint application of these methods enable a spatially precise characterization of tissue perfusion across its gray matter and subfields, in particular. For our second goal, we assess the impact of nearby arterial structures reconstructed using a high-resolution TOF-MRA on the perfusion maps. And finally, by leveraging the harmonized unfolded space, we will assess the cross-correlation between hippocampal perfusion, morphometry, other MRI-based properties as well as cytoarchitectonic features extracted from a histological hippocampal sample provided by the BigBrain project (34, 35). Altogether, the presented results provide significant neuroscientific findings that will aid the community to interpret hippocampal (subfield) changes relevant in the context of neurological diseases and/or cognitive neuroscience, as well as an imaging framework that can be used to guide researchers in setting up protocols and analysis of such data to study hippocampal perfusion.
Results
Perfusion in the Hippocampus.
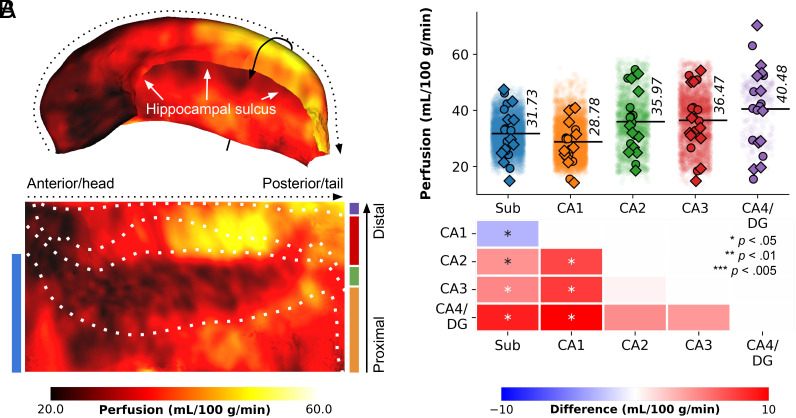
Surface mapping and unfolding of the high-resolution ASL data demonstrate that the hippocampal gray matter is characterized by a spatially varying perfusion pattern. The average perfusion map across all 8 runs, 10 subjects and both hemispheres (i.e., totaling to an average of 160 perfusion maps) depicted in Fig. 1A, showcased the following patterns: a) lower perfusion in the anterior portion (hippocampal head) and along the hippocampal sulcus (white arrows), b) higher perfusion toward the posterior portion (hippocampal tail) and both proximally and distally (solid arrow). Note that the proximal–distal (i.e., transverse) axis is defined with respect to its boundary with the neocortical tissue (36) instead of the DG, as in rodent and non-human primate literature (37).
Fig. 1.
Perfusion mapping in the hippocampus. (A) The figure displays perfusion values (mL/100 g/min) mapped on folded and unfolded hippocampal surfaces. The dotted and solid arrows indicate the anterior–posterior and proximal–distal (from neocortical tissue) axes, respectively. Subfield boundaries, derived from cytoarchitectonic features of the BigBrain atlas, are overlaid on the unfolded map. (B) Subfield averages, color-coded based on the subfield atlas overlaid on maps in A, are presented for each subject and hemisphere (circles for the left hemisphere, diamonds for the right hemisphere), as well as per vertex (semi-transparent dots averaged across subjects and hemispheres). Pairwise comparisons between subfield averages are depicted as heatmaps, with FDRBH-corrected P-values indicated by asterisks: *P< 0.05, **P< 0.01, ***P< 0.005.
To facilitate interpretation, the perfusion map was mapped onto the canonical unfolded hippocampal surface, with the Sub, CA1, CA2, CA3, and CA4/DG subfields arranged from bottom to top, and the head, body, and tail aligned from left to right. Friedman’s tests for repeated measures, based on subject-wise subfield averages, demonstrated significant differences in perfusion values among subfields ((4) = 19.68, P< 0.001, Fig. 1B). Particularly, CA1 exhibited significantly lower perfusion (average of 28.78 mL/100 g/min) compared to the other subfields (pFDR< 0.05), as indicated by pairwise comparisons (refer to heat maps in Fig. 1B). Perfusion levels in CA2, CA3, and CA4/DG did not exhibit significant variations among each other. These findings broadly align with Duvernoy’s seminal work (21). For interactive exploration of vertex- and subfield-wise data, we direct the reader to our online app.*
Reliability of hippocampal perfusion estimates.
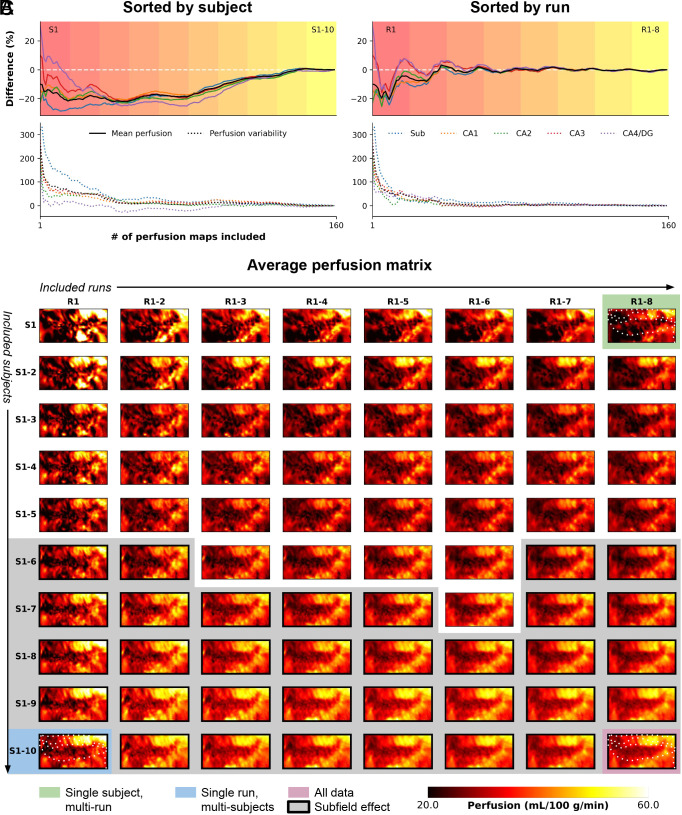
Fig. 2 illustrates the evolution of the perfusion map in the hippocampus, providing insights into the minimum required number of runs and/or subjects for future studies. We evaluated the stability of average perfusion and the variability, assessing the coefficient of variation (CoV) for perfusion values (i.e., homogeneity), across vertices in each subfield, with varying subject (S1–10, Fig. 2A) and run (R1–8, Fig. 2B) quantities (in consecutive order). Fig. 2C presents the unfolded representation of vertex-wise perfusion estimates as a function of included runs (columns) and subjects (rows), with thick outlines and gray background indicating significant subfield effects (P< 0.05 based on the Friedman’s test). Notably, this visual representation demonstrates a gradual transition toward the final perfusion pattern, with discernible subfield effects observed from six subjects onward. Two key observations emerge from this analysis: a) the stability of the mean perfusion signal (solid lines) is largely influenced by the number of subjects in the cohort, and b) perfusion variability (dotted lines) tends to deviate more than the mean perfusion signal, particularly with smaller amounts of included data.
Fig. 2.
Evolution of high-resolution perfusion maps. The figure illustrates the progression of high-resolution perfusion maps, showcasing the percentage difference in mean perfusion (solid lines, Top) and variability (dotted lines, Bottom) across the entire hippocampus (in black) and individual subfields (color-coded). The evolution is presented as a function of (A) the number of included subjects or (B) runs. (C) Additionally, the figure depicts the unfolding of the average perfusion map and its evolution as a function of the number of included runs (rows) and subjects (columns) in consecutive order.
As expected, the findings in Fig. 2 A and B emphasize the critical role of cohort size and data inclusion in achieving stable and reliable hippocampal perfusion measurements, shedding light on the interplay between stability, variability, and the quantity of included data. Furthermore, they reveal variations in the evolution of perfusion estimates depending on the sorting criteria, either subject-wise or run-wise. Hence, we performed additional bootstrapping to mitigate potential sampling biases. Heat maps in SI Appendix, Fig. S1 show the median across iterations relative to the results obtained from the full fit analysis based on the 160 perfusion maps, indicated by the black round marker in A, which corresponds to the maps presented in Fig. 1. The results confirm a lower dependency of mean perfusion estimates on the amount of included data (up to 1% difference, SI Appendix, Fig. S1A) compared to between-vertex variability (up to 100%, SI Appendix, Fig. S1B). Notably, the number of included subjects exerts the strongest impact. In contrast, perfusion temporal SNR (tSNR) exhibits a gradual stabilization with increasing runs, rather than subjects (SI Appendix, Fig. S1C). Consistent with improved subfield homogeneity (Fig. S1B), the between-subfield effect size gradually increases as more subjects are included (SI Appendix, Fig. S1D), with a significant effect (P < 0.05) already observable starting from three subjects.
The inter-subject differences in Figs. 1B and 2 are in line with the large perfusion differences observed previously across healthy individuals (38). This leads to relatively large CoVs across subjects and hemispheres per subfield (30.3% on average). Nonetheless, taking into account the variability in mean hippocampal gray matter perfusion differences between subjects (SI Appendix, Fig. S2) significantly reduces the CoV to 11.7% (paired Student’s t test, t(4) = 21.10, P< 0.001), while the significant subfield effect across all subjects and hemispheres is maintained ((4) = 20.88, P< 0.001).
Characterization of MRI Quality and Morphometric Hippocampal Features.
To assess the influence of other hippocampal properties on the perfusion pattern, we mapped different acquisition- and morphology-related metrics. The average tSNR of hippocampal perfusion was 3.35 ± 0.84 (SI Appendix, Fig. S3A). We found an average of 12.03 ± 1.15 T across all data points (SI Appendix, Fig. S3B). We have previously estimated that a threshold of 6.54 T is required to meet the adiabatic condition for our tr-FOCI inversion pulse (39). Susceptibility-induced distortions in perfusion imaging were most prominent in the anterior portion (hippocampal head), peaking at 0.3 mm within the Sub and CA1 (SI Appendix, Fig. S3C), consistent with their proximity to the air-tissue interface. However, the magnitude of these distortions was relatively modest compared to those typically observed in functional or diffusion MRI (40, 41).
For validation purposes, we also quantified additional hippocampal tissue properties, including morphology-related metrics such as cortical thickness, curvature, and gyrification derived using HippUnfold, as well as metrics related to underlying tissue microstructure, predominantly reflecting myelin content based on T1w/T2w ratio maps. Each metric displayed distinct spatial patterns (SI Appendix, Fig. S4). Cortical thickness was lowest in CA2, while gyrification was most pronounced along CA1 toward the head. The subiculum exhibited the strongest myelination, as indicated by low T1 values but high T1w/T2w ratios. These findings align with previous observations, confirming the expected variations in hippocampal tissue properties across subfields (33).
Tracing Hippocampal Vascularization.
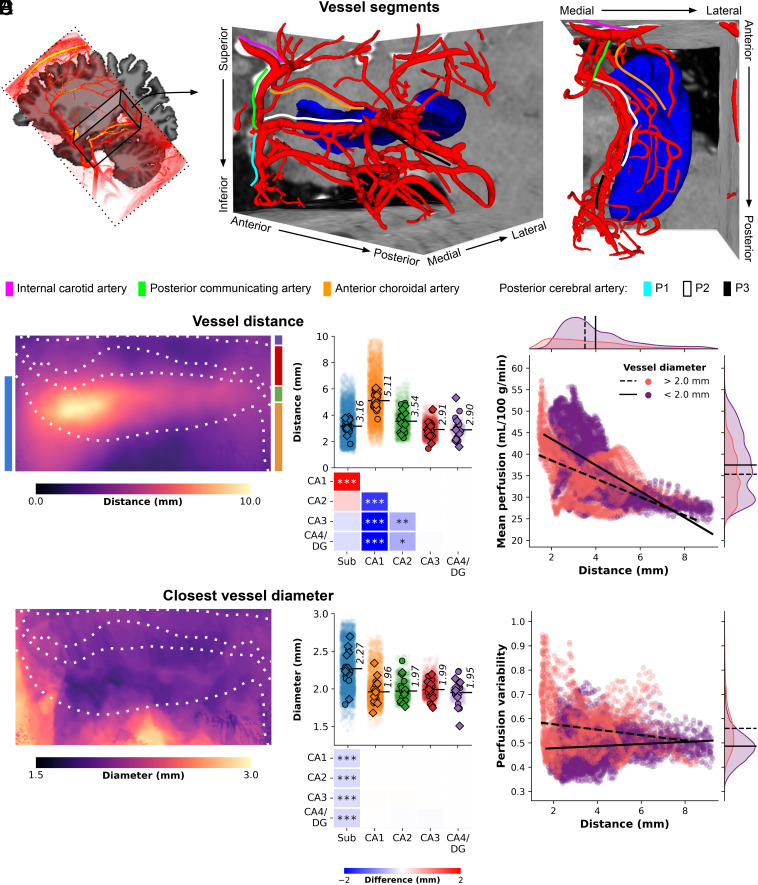
In our second objective, we utilized high-resolution TOF-MRA data to reconstruct the macrovasculature of the hippocampus for combined analysis with the perfusion data. Fig. 3A illustrates a 3D reconstruction example of the right hippocampal macrovasculature for a single subject (see SI Appendix, Fig. S5 for an overview of all subjects). The topology of the reconstructed vasculature aligns closely with previously identified patterns and trees of hippocampal vascularization (24). The prominent internal carotid artery (ICA, depicted by magenta solid lines) serves as the primary blood supply source to the medial temporal lobe. The posterior communicating artery (PCA) connects the ICA with the P1 (cyan) and P2 (white) segments of the posterior cerebral artery, with the latter running parallel to the anterior–posterior axis of the hippocampus. Similar to the PCA, the anterior choroidal artery (orange) arises from the ICA and follows a superior position in the same direction. Interactive visualizations of these 3D reconstructions, as well as additional representations such as node-wise networks and vessel geometry properties (including ), specific to each subject’s hippocampus, can be accessed in the interactive HTML notebooks provided in the online code repository.† Collectively, these visualizations demonstrate that the network of interconnected arteries described above was identifiable in most cases. However, the detection of thinner arteries, such as the anterior and posterior hippocampal arteries, was less reliable across subjects due to their diameter falling below the effective resolution of the TOF-MRA data.
Fig. 3.
Hippocampal vasculature and perfusion relationship. (A) Three-dimensional reconstruction of a subject’s macrovasculature in close proximity to the right hippocampus, showcasing delineated vessel segments. (B) Hippocampal vessel distance (mm) depicted on an unfolded hippocampal surface. Strip plots display color-coded subfield averages for each subject, including left hemisphere (circles) and right hemisphere (diamonds), along with per-vertex values (i.e., averages across subjects and hemispheres shown as semi-transparent dots). Heatmaps illustrate pairwise comparisons between subfield averages, with FDRBH-corrected P-values indicated by asterisks: *P< 0.05, **P< 0.01, ***P< 0.005. (C) Similar to (B), but representing vessel diameter (mm) of the nearest vessel. (D) Scatter plot illustrating the relationship between vertex-wise mean perfusion (mL/100 g/min) and the shortest distance to a vessel (mm), stratified by respective vessel diameter (color-coded as thinner or thicker than 2 mm). Linear fits for each group are depicted by solid and dashed black lines. (E) Similar to (D), but contrasting with perfusion variability determined by the coefficient of variation across all maps (i.e., across runs and subjects).
Linking hippocampal vascularization and perfusion.
Once the vessel tree for each subject was established (Fig. 3A), vessel-related metrics were projected onto the hippocampal surfaces to examine the positioning of vertices and subfields relative to the hippocampal vasculature (refer to SI Appendix, Fig. S6A for an example of the metrics). It is important to note that the presented diameter values are estimates limited by the spatial resolution of the TOF-MRA data, which hinders the reliable identification of vessels smaller than 0.5 mm. The averaged results across subjects highlight variations in the distance to vessels throughout the hippocampus, ranging from 0 to 10 mm, with differences observed between subfields ((4) = 25.2, P < 0.001, Fig. 3B). Specifically, CA1 is located farthest from the vessels, with an average distance of 5.11 mm, while the subiculum (3.16 mm), CA3 (2.91 mm), and CA4/DG (2.90 mm) exhibit closer proximity to macrovasculature structures. Notably, the vessel distance map (Fig. 3B) demonstrates a distinct pattern along the anterior–posterior axis of the hippocampus. Moreover, vessels in close proximity to the subiculum (e.g., PCA) tend to have relatively larger diameters (average of 2.27 mm) compared to vessels near other subfields ((4) = 21.4, P < 0.001, Fig. 3C). An important finding from this analysis is that the largest perfusion values are associated with the proximal vasculature (Fig. 3D), and overall perfusion signals remain relatively stable across different vessel sizes and their distances from hippocampal tissue (Fig. 3E). Characterization of the differences between the perfusion signal within the hippocampal GM and vessels indicate that the majority of labeled blood did reach the capillaries (SI Appendix, Fig. S7). This is strengthened by the negative perfusion-weighted signal within vessels, which implies the mixing with fresh (uninverted) blood during TI1. Nonetheless, the observed residual label could contribute to the larger hippocampal perfusion values more close to the macrovasculature.
Quantification of Hippocampal Features Cross-Correlation.
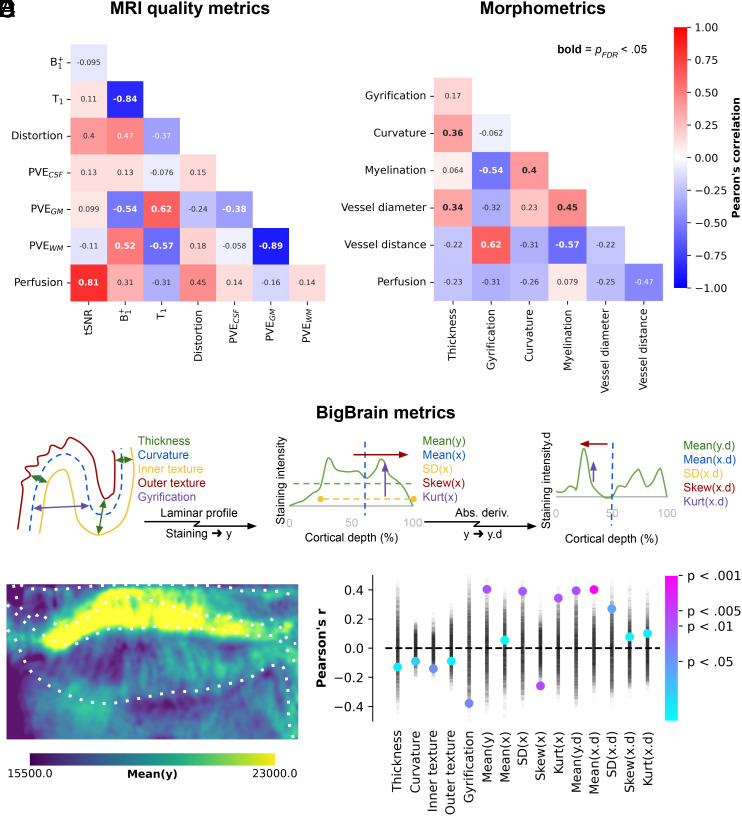
Having demonstrated that perfusion levels vary across hippocampal gray matter, with higher perfusion levels linked to a closer distance to vascular structures, and that we have confirmed previously established patterns for its morphometry (thickness, gyrification, and curvature) and myelination (36), we set out to map out their interdependencies. To accomplish this, we computed the Pearson’s correlation coefficient to assess the similarity among all pairs of hippocampal features and their vertex-wise averages (Fig. 4A). Perfusion did not demonstrate significant correlations with image distortion, B, T1, and partial volume estimates (Proll> 0.05, Fig. 4A). However, a significant correlation was observed with tSNR (Pearson’s r = 0.81, Proll< 0.001, Fig. 4A), indicating that higher perfusion values were obtained in regions less dominated by noise. It is reassuring that our measurements and thus findings of hippocampal perfusion patterns appear robust to acquisition-related metrics.
Fig. 4.
Between-feature correlations. (A and B) Heatmaps depicting the correlations between different features (SI Appendix, Figs. S3 and S4) on their vertex-wise averages, with corresponding Pearson’s correlation coefficients annotated. Significant correlations, after correcting for spatial autocorrelation and multiple comparisons, are indicated by bold annotations. Panels (C–E) illustrate the correlations between perfusion and various hippocampal morphometric and staining intensity-based features derived from the BigBrain sample. In panel (E), the point plot displays permuted Pearson’s correlation coefficients represented by semi-transparent black markers, which were used to calculate color-coded significance levels.
Regarding the two macrovascular features, we found the strongest correlation between perfusion measures and distance to vessels (Pearson’s r = −0.47, Proll = 0.06), indicating that regions further away from vessels tend to exhibit lower perfusion (Figs. 1A and 3E). The impact of vessel diameter on measured perfusion was relatively smaller, consistent with the notion that smaller vessels, which are more relevant to tissue perfusion, are typically in closer proximity to the hippocampal gray matter (Fig. 3D).
One hypothesis for the relatively modest correlation between perfusion and the other (morphometric) features might be that perfusion levels are weighted stronger toward local differences in metabolic demand. To explore this further, we repeated the correlation analyses using cytoarchitectonic features derived from the BigBrain model (Fig. 4C) (35, 36). As they are derived from a histological staining for cell bodies, these cytoarchitectonic measures provide insights into the distribution of cell bodies within the hippocampal gray matter across its three axes (anterior–posterior, proximal–distal, and cortical depths) and can serve as proxies for variability in metabolic activity (i.e., heightened activity and functional requirements). Fig. 4D shows an example of one of these features, linked to the mean staining intensity (i.e., derivative of mean Y) per hippocampal vertex. Among all tested features, perfusion appears most strongly (i.e., significantly, Proll< 0.05) correlated with the hippocampus’ cytoarchitectonic rather than its morphometry aspects (Fig. 4E), suggesting a stronger dependence of perfusion on laminar features and possible associations with metabolic demand.
Discussion
Perfusion in the Human Hippocampus.
The objective of this study was to establish an imaging framework that enables users to accurately assess variations in hippocampal perfusion among its subfields. Here we show that it is possible to acquire robust perfusion-weighted data with consistent slab positioning across all subjects (SI Appendix, Fig. S8 A or B) for high-resolution (1.5-mm isotropic) perfusion quantification using ASL at 7T. The perfusion measures, averaged across our cohort, fell within the expected physiological range in healthy humans (SI Appendix, Fig. S8C) (42, 43). For reference, the perfusion in the visual cortex was V1: 58.24 ± 15.68 mL/100 g/min, V2: 44.42 ± 10.91 mL/100 g/min. The quantitative perfusion values observed in the hippocampus, although lower than those in V1 and V2, are unlikely to be artifactual based on the robustness of our data. Instead, they are likely attributed to the relatively lower microvascular density, which serves as the source of our perfusion signal, in the hippocampus compared to neocortical tissue (like V1 and V2) (44, 45). We demonstrate that there are clear, measurable differences between subfields. Most strikingly, CA1 appears to be characterized by the lowest perfusion among hippocampal subfields, which is in line with previous in vivo and ex vivo indices of microvascular density in animals (46) and humans (25, 47). While characterized by a lower microvascular density and blood flow, CA1 is not necessarily characterized by a difference in activity due to the prominent role of its (mostly pyramidal) neurons in hippocampal structure and function (48). This thus renders CA1 particularly vulnerable in case of metabolic insults and confirms its observed higher susceptibility across several diseases (14, 49). Furthermore, our stability analyses have demonstrated that the observed perfusion pattern stabilizes quickly and can be reliably detected with a relatively small sample size of six subjects and a total ASL scan time of only five minutes per subject (∼50 perfusion-weighted images). These findings indicate that a general-purpose high-resolution ASL protocol at 7T, as employed in this study, is capable of providing sufficient perfusion information in medial-inferior cortical regions like the hippocampus. Therefore, it suggests that specific optimization tailored to each region is not necessarily required (42, 50). However, it is worth noting that the above recommendation was based on data obtained from healthy and experienced control subjects. For researchers, particularly those investigating hippocampal perfusion in clinical populations, we advise acquiring as much ASL data as feasible within the available scan time to ensure comprehensive analysis and accurate interpretation of the findings.
Vascularization and Its Impact on Hippocampal Perfusion.
Based on the aforementioned considerations, one may reasonably attribute the relatively diminished perfusion observed in the CA1 region and its heightened susceptibility to disease to its comparatively lower microvascular density. However, it is likely that the observed differences in perfusion across the hippocampal subfields were not only impacted by the density of small blood vessels but also by their proximity to the nearby macrovasculature. This intricate network of arteries and vessels supplies oxygen and nutrients to the hippocampal tissue and supports its metabolic demand and proper functioning (51). The two primary arteries involved in hippocampal perfusion are the posterior cerebral arteries (PCAs) and the anterior choroidal arteries (ACHAs). However, the vasculature of the brain is highly interconnected, and there may be additional contributions from other arteries to hippocampal perfusion, including the hippocampal branches of the middle cerebral arteries (MCAs) (51). We employed TOF-MRA to map subject-specific vessel branching patterns around the hippocampus in vivo, generating reconstructions consistent with previous descriptions of hippocampal vascularization (23, 24, 51). Our reconstructions, along with joint analyses of perfusion estimates, suggest that the subiculum’s perfusion is most likely provided by collateral branches of the PCA’s P2 segment—a vessel that runs parallel to the anterior–posterior hippocampal axis and exhibits a larger diameter. Most importantly, these results suggest that the lower perfusion in CA1 might indeed be partly ascribed to its further distance from the macrovasculature, especially toward the hippocampal head. It should be noted that residual labeled blood within the macrovasculature can impact the observed hippocampal perfusion patterns as well. Hence, it is possible that increased partial voluming of perfusion-weighted signals between arteries and the subiculum, CA2, CA3, and CA4/DG might have artificially elevated their perfusion estimates. However, the dominance of gray matter tissue contributions observed in the perfusion analyses decreases the likelihood of this scenario (52). Combining measurements of both distance and diameter demonstrates that the relationship between mean perfusion and distance is strongest when considering smaller vessels (i.e., <2 mm), whereas increased variability in perfusion across subjects is more closely associated with the closer proximity of relatively larger vessels (i.e., >2 mm). These integrative analyses collectively indicate that macrovascular structures likely influence the measured perfusion pattern and introduce variability in hippocampal perfusion measurements among subjects. Therefore, it is advisable for future work to compare hippocampal perfusion maps between groups of subjects characterized by different hippocampal vascularization patterns to gain insights into the observed differences, particularly in the context of disease (23, 53).
Methodological Aspects of Quantifying Hippocampal Perfusion.
While we have successfully demonstrated the feasibility of reliably characterizing hippocampal perfusion, it remains a challenging task that necessitates certain expertise to ensure high-quality data. In this study, we implemented a multi-modal, multi-resolution acquisition protocol for 7T MRI. The use of 7T MRI offers improved image quality compared to 3T MRI, thanks to increased SNR (32) and potential enhancements in spatial resolution. This enhancement allows for better anatomical delineation of hippocampal subregions (54) and improved sensitivity to perfusion differences (55). However, the inclusion of scans with small field-of-view and different orientations introduced an additional challenge in terms of data integration. This challenge becomes evident when overlaying the slab positioning for the various acquisitions (refer to SI Appendix, Fig. S9). Nevertheless, not all of these scans are equally critical. In the following discussion, we address this aspect and propose a set of minimal requirements to be considered when conducting hippocampal perfusion imaging, ensuring feasibility and data quality.
For anatomical imaging, we recommend acquiring at least an MP2RAGE image (56) and a map [e.g., using the Sa2RAGE sequence (57)] to improve hippocampal T1 quantification (58, 59), which subsequently enhances the precision of voxel-wise perfusion estimates (60). Consistency in subfield labels and harmonization across subjects is crucial to maximize the spatial specificity of hippocampal perfusion maps (4). Therefore, in this study, we opted to acquire additional T2-weighted images to extract hippocampal surfaces and perform subfield parcellation using the HippUnfold analysis suite (33). The Hippocampal Subfields group suggests the use of T2-weighted images for manual segmentation of the hippocampus because of their optimal contrast between the hippocampal gray matter and stratum radiatum and lacunosum-moleculare (SLRM) tissue (29, 61). SI Appendix, Fig. S8 D–G provides an example of manual segmentation and corresponding surface representation for the left and right hemispheres of a single subject. While T2-weighted images are generally preferred, recent advancements in HippUnfold enable precise and automatic segmentation even when only T1-weighted data are available (33). Furthermore, although the CA4 field and DG are distinct anatomical entities (3), they were combined into a single label due to their limited size. However, in the most recent releases of HippUnfold (v1.0.0 and newer), the DG is modeled as a separate surface to increase specificity.
Furthermore, we would like to emphasize several aspects regarding our perfusion imaging protocol. ASL-based perfusion imaging is a B sensitive technique and therefore challenging to acquire at 7T due to its transmit field inhomogeneities, especially toward the lower part of the brain (e.g., inferior frontal and temporal lobes) (62). This consideration is important to note when transitioning from 3T to 7T for perfusion imaging. To address this, we employed dielectric pads (39, 63) and an optimized inversion [tr-FOCI (64)] pulse to achieve higher labeling efficiency (i.e., =0.95) in the hippocampal region (SI Appendix, Fig. S3B) and the adjacent vasculature (SI Appendix, Fig. S6C) for all subjects(31, 39). Last and more generally, reducing geometric distortions, high-spatial resolution, and isotropic voxels are crucial to reduce partial voluming effects and thereby, improve the perfusion CNR (65). Some ASL protocols employ 3D-GRASE readouts to obtain relatively higher SNR (66) but they come at the cost of increased blurring in the z-direction (67) as well as higher SAR at ultra-high field strengths. Alternatively, ASL with spiral (68) and 3D-EPI (69) readouts have shown promise to enable high-resolution, SAR efficient perfusion imaging at ultra-high fields. In this study, we did not set out to optimize the PLD parameter for hippocampal imaging in particular due to our cohort consisting of young, healthy participants and to prevent erroneous estimation of hippocampal gray matter perfusion (70). However, this should be considered when imaging other cohorts such as healthy elderly or patients, as the longer arrival times necessitate increasing PLD to obtain robust perfusion (71).
Clinical Applicability of Hippocampal Perfusion Imaging.
Alterations in hippocampal perfusion have been observed in various diseases such as Alzheimer’s disease (72), temporal lobe epilepsy (73), and schizophrenia (74). However, these findings have primarily relied on imaging techniques such as positron emission tomography, single-photon emission computed tomography, or ASL with limited spatial resolutions (i.e., >2.5 mm isotropic) and, hence, did not allow quantification of subfield-specific perfusion changes. While such precise assessments become feasible using the presented 7T MRI framework, the utility of 7T MRI is currently limited to specific clinical indications, such as 3T MRI-negative focal epilepsy patients (75). Nonetheless, this is likely to improve with the growing availability of FDA- and CE-approved 7T MRI scanners.
There were some additional considerations when we were designing our high-resolution imaging protocol. A most important aspect for perfusion studies using ASL is the motion between consecutive control and label images (the voxel-wise subtraction is no longer consistent). In order to be more robust to motion and minimize data loss, our protocol entailed several five-minute ASL scans. From our experience, this is better tolerated by elderly/patient populations, even at 3T, than a single longer scan. We used a single-shot EPI acquisition that is inherently less sensitive to motion than multi-shot/segmented methods (76). Furthermore, the high resolution of our data, experienced participant cohort together with a repetition time (TR) of 2.861 s enabled us to compensate for motion in post-processing without requiring complicated methods that need advanced sequence development such as using navigators, or using in combination technologies such as prospective motion correction in the scanner (77), especially at 7T. We have not systematically evaluated the interaction effects of motion and resolution, however, given our spatial resolution, single-shot 2D-EPI readout, and functional MRI (fMRI)-like TR of 2.861 s, we expect motion tolerance to be similar to fMRI where typically 1 to 2 voxels (∼2 to 4 mm) millimeters is considered a threshold for censoring volumes (78). Please note, this may not apply for other ASL acquisitions using segmented 3D readouts. Finally, in the context of ASL, having higher resolution means having more voxels of a tissue type that share a neighbor with the same tissue type. Therefore, the potential for cross-tissue contamination due to motion is much lower than standard clinical ASL scans (79). Building on the technical advances at 3T, future studies can address translation of these findings to standard clinical field strengths in elderly and patient cohorts.
Concluding Remarks and Outlook.
By quantifying blood flow across hippocampal subfields, we can gain a better understanding of the normal patterns of perfusion and how they relate to the specific functions associated with each subfield. Here, we presented and validated a 7T MRI imaging framework that allows in vivo characterization of perfusion differences across the hippocampus. Our hippocampal perfusion map can serve as a baseline for comparison with diseased states, where it possibly allows for early detection and/or assessment of disease progression in individuals with hippocampal-related disorders. Diseases that cause even modest reductions in hippocampal blood flow, potentially due to capillary rarefaction, hyperconstriction, and inward remodeling of hippocampal arterioles, would likely have a tremendous impact on neuronal function, memory, and cognition (80). Finally, future studies using advanced TOF-MRA acquisition and analyses (81) are warranted to precisely link differences in hippocampal perfusion to differences in macrovascular anatomies among individuals.
Materials and Methods
Eleven healthy volunteers (mean age 26±3.2 y, five males) participated in this study after having provided written informed consent. The study was approved by the Ethics Review Committee Psychology and Neuroscience (ERCPN) at the Faculty of Psychology and Neuroscience, Maastricht University, The Netherlands, and all procedures followed the principles expressed in the Declaration of Helsinki.
Data Acquisition.
All data were acquired on a Siemens Magnetom 7T scanner (Siemens Healthineers) with an SC72 whole-body gradient system capable of a maximum gradient amplitude of 70 mT/m, maximum slew rate of 200 T/m/s using a 1Tx/32Rx phased array head coil (Nova Medical) housed at Scannexus B.V. The participant preparatory and positioning procedure followed the protocol previously described in refs. 31, 55, and 69. Briefly put, the center of the eyes was used as the iso-center reference (instead of the eyebrows, as is typically done), supplemental cushions were provided to the participants under the neck, to ensure that the large feeding arteries to the brain were as close to parallel to the B0 as possible. In addition, two 13 × 13 × 0.5 cm3 high-permittivity dielectric pads containing a 2.8:1 solution of calcium titanate (CaTiO3) in heavy water (D2O) by weight (82) were placed on either side of the neck to improve the B (therefore, labeling) efficiency at 7T (63). In six participants, a third dielectric pad was placed over the participant’s right lateral side to reduce the impact of the hemispheric asymmetry of the coil’s inherent profile (39).
Anatomical data.
A whole-brain 3D-MP2RAGE (56) dataset at 1.0 mm isotropic resolution was acquired first and used to inform slice positioning during the rest of the session. A 3D-Sa2RAGE (57) dataset at 2 mm isotropic was acquired to facilitate correction of the T1 maps (83). At least three repetitions of an ultra-high-resolution 0.4 mm in-plane resolution T2-weighted 2D-TSE (84) were acquired using oblique coronal slices positioned to cover the entire hippocampal complex bilaterally. Two ultra-high resolution 0.5 mm isotropic 3D-MP2RAGE scans with a partial coverage (entire hippocampal region axially) were acquired. Due to SAR constraints, 2D-TSE scans could not be acquired consecutively, the 0.5 mm 3D-MP2RAGE scans were interspersed between the 2D-TSE scans for time efficiency. Finally, two repetitions of ultra-high resolution 0.5 mm isotropic 3D multi-slab time-of-flight (85–87) MR angiograms were acquired (3D-TOF-MRA). Complete sequence details are tabulated in Table 1.
Table 1.
MRI acquisition details
| Anatomy | Perfusion | Vasculature | |||
|---|---|---|---|---|---|
| Parameter | MP2RAGE | TSE T2w | ASL | TOF-MRA | |
| TR (ms) | 6,000 | 6,000 | 9,000 | 2,861 | 15 |
| TE (ms) | 1.88 | 3.98 | 105 | 14 | 3.59 |
| TI1/TI2 (ms) | 800/2,750 | 983/2,940 | 700/1,800 | ||
| FA1/FA2 () | 4/5 | 6/7 | 132 | 70 | 15 |
| GRAPPA | 4 (A-P) | 2 (A-P) | 2 (F-H) | 3 | 3 (F-H) |
| No. of slices | 192 | 72 | 50 | 32 | 220 |
| Slice direction | Sagittal | Sagittal | Coronal | Sagittal | Coronal |
| Field of view (mm) | 256×256 | 184×184 | 192×192 | 192×192 | 210×210 |
| Matrix size | 256×256×192 | 368×368×144 | 512×512×50 | 128×128× | 448×448×x440 |
| Resolution (mm) | 1×1×1 | 0.5×0.5×0.5 | 0.4×0.4×1 | 1.5×1.5×1.5 | 0.5×0.5×0.5 |
| Phase partial Fourier | 6/8 | Off | 6/8 | 6/8 | |
| Slice partial Fourier | Off | 6/8 | 6/8 | ||
| Bandwidth (Hz/px) | 250 | 140 | 90 | 1698 | 203 |
| Acquisition time (m:s) | 7:14 | 5:26 | 4:41 | 4:49 | 5:50 |
| Number of runs | 1 | 2 | 3 | 8 | 2 |
Total acquisition time 1:20:21 (h:m:s).
See SI Appendix, Fig. S9 for a schematic of the scanning order and positioning of the imaging slabs.
Perfusion data.
Perfusion data were acquired at 1.5-mm isotropic resolution using a Pulsed Arterial Spin Labeling (PASL) sequence (88) employing a FAIR (89) QUIPSS II (90) labeling scheme with a 2D-EPI readout. For each participant, eight consecutive runs of 50 control-label repeats (i.e., 100 volumes) were acquired with each run lasting ±5 min. An equilibrium magnetization (M0) image was acquired using the same 2D-EPI readout, but with no magnetization preparation and the TR increased to 20 s. A second M0 image was acquired immediately after with the opposite phase-encoding direction for distortion correction.
Data Pre-Processing.
All stages of data pre-processing and registrations outlined below were subject to careful visual inspection for quality control. Please see SI Appendix for technical details of each step.
Anatomical data.
The TSE runs were first resampled to 0.3 mm isotropic resolution and a minimally deformed average TSE template was created from the 0.3 mm TSE datasets using ANTs.‡ This resampled 0.3 mm isotropic TSE template image was used for manual hippocampal segmentation and was defined as the final reference space for co-registering all other image modalities in the present study. Both whole-brain (1 mm3) and high-resolution (0.5 mm3, “hires-”) MP2RAGE datasets were corrected for transmit efficiency () inhomogeneities using a separately acquired Sa2RAGE map (57) in line with (83). The -corrected MP2RAGE UNI images were then background denoised and pre-processed using presurfer§ (91) and were processed using the recon-all pipeline for cortical segmentation and surface reconstruction in Freesurfer 7.1.1(92).¶ The cleaned hires-UNI, corrected hires-UNI images and hires-T1 maps were resampled to 0.3 mm isotropic resolution, averaged and used for further analyses. The TOF-MRA MRA data were resampled to 0.3 mm isotropic resolution, co-registered using greedy# (93) and averaged. This average 0.3 mm isotropic TOF-MRA image was used for vascular segmentation.
Perfusion data.
First, the blip-up and blip-down M0 images were processed with FSL to estimate the phase-encoding distortion correction using FSL’s topup (41). All ASL images were motion-corrected to the M0 as a reference and all ASL runs were corrected for motion and phase-encoding distortions using a single resampling step using FSL. Perfusion-weighted images (PWI) were calculated from the ASL timeseries datasets using the surround-subtraction (94, 95). Perfusion temporal signal-to-noise (tSNR) map was calculated by dividing the PWI temporal mean by the PWI temporal SD images. Perfusion quantification was carried out in native space using a single, well-mixed tissue compartment model (i.e., the Buxton model) (96) as implemented in oxasl‖ (60, 97). The following parameters were modified as per our acquisition scheme [inversion efficiency = 0.95, (55)] and the field-strength [T1,blood = 2.2 s, (98); T1, tissue = 1.8 s (40, 83, 99)]. All processing stages were quality controlled using visual inspection as well as using the automatic report generated by oxasl that includes the model fitting and quantification stages. This led to one out of eleven subjects to be excluded from further analyses. Besides perfusion tSNR, other metrics for perfusion MRI quality control included partial volume estimates (PVE) for gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissue classes, as well as B, T1 and susceptibility distortion-related deformation images (i.e., topup output).
Co-registration among image modalities.
Prior to any further analyses, the MP2RAGE, TOF-MRA, and perfusion data were co-registered to the 0.3-mm TSE template image using workflows optimized for each image modality as described in SI Appendix.
Hippocampus and Subfield Segmentation.
The 0.3 mm isotropic average TSE data were used to manually segment the hippocampus for each subject (SI Appendix, Fig. S8D). In the average TSE data, the contrast between the stratum radiatum and lacunosum-moleculare (SLRM), or “dark band,” and the neighboring hippocampal GM tissue is improved and was essential to facilitate manual segmentation. First, individual masks for both SLRM and GM tissues were created semi-automatically using the active contour segmentation mode in ITK-SNAP v3.8.0 (100) and were manually edited following the recommendations in ref. 36. Additionally, several “boundary” labels were added to encode for the anterior–posterior (A-P), proximal–distal (P-D), and inner–outer (I-O) axes (Fig. S8E).
Following the manual segmentation, each hippocampus was unfolded using the snakemake (101) implementation of our in-house developed hippocampal unfolding tool (SI Appendix, Fig. S8 F and G) (33). In brief, this method entails the following steps: i) alignment of the subject-specific T2w image and its manual segmentation to the coronal oblique atlas space, ii) imposing coordinates along the A-P, P-D and I-O dimensions onto the hippocampal GM by solving the Laplace equation, iii) extracting inner, mid-thickness and outer GM surfaces while ensuring one-to-one vertex correspondence between them, and iv) estimating the native-to-unfolded space transformation to analyze data in a common 2D plane. A detailed description of the unfolding algorithm can be found in the original work (36) and online documentation.** All the surface-based output was generated within the GIfTI framework to allow easy manipulation, volume-to-surface mapping (see following sections) and visualization using Connectome Workbench (102). Exploration of the manual segmentations and HippUnfold output is possible using the HTML visualization notebooks provided in the online code repository.
Vascular Segmentation and Reconstruction.
The average 0.3 mm TOF-MRA data were used to identify macrovascular structures within the vicinity of the hippocampus. First, the TOF-MRA image was spatially filtered using non-linear anisotropic diffusion (103, 104) by exploiting the structure tensor field derived from the images as implemented in Segmentator v1.6.0 (105).†† This preserves the boundaries between vessels and brain tissue while reducing intra-tissue class image noise. Next steps were carried out in MeVisLab v3.3 (106).‡‡ First, vessel-like structures were extracted from the “smoothed” TOF-MRA image for 3D reconstruction. Then, for each subject and hemisphere, the input image was rescaled to range between 0 and 100 a.u. (i.e., to match intensity ranges across subjects), then thresholded to increase the contrast between vessels and background (i.e., GM and WM, image intensity <10 a.u.) tissue and finally used to manually define ±150 seeding points to segment connected vessels. This ensures that all voxels connected in the x, y or z direction with a seed point, and within the specified intensity range will be segmented. Here, the lower threshold was optimized for each subject based on manual inspection of the vascular tree after its automatic 3D reconstruction. This was achieved by a) extracting the vessels’ skeleton based on the centerline of the binary segmentation label, b) transforming the skeleton into a graph to encode geometrical and structural shape properties so as to allow c) the decoding of the graph properties into a polygonal surface of the vascular tree for 3D visualization (107). Finally, surfaces were transformed to voxel-wise representations and skeleton graphs saved as an XML file for network reconstruction and analyses.
Data Integration and Visualization.
Subsequently, the hippocampal mid-thickness surface, ASL, and TOF-MRA output maps were combined to assess their relationship. First, for each hippocampal GM voxel, its vessel distance (in mm) was calculated by finding the Euclidean distance to the closest boundary voxel of any vessel (108). These and the diameter of the respective closest vessel segment for each hippocampal GM voxel were then stored as NIfTI volumes using nibabel v3.2.0 (109). See also SI Appendix, Fig. S6A for a schematic demonstrating this procedure. Second, for the co-registered imaging data, vessel distance, and closest vessel diameter images, Connectome Workbench’s -volume-to-surface-mapping command-line tool was used to sample along the hippocampal GM mid-thickness vertices, hereby constraining the mapping algorithm to only include voxels that were labeled as GM and found between the inner and outer GM surfaces. As such, each vertex’s value represents a weighted average of the voxels along the IO dimension with lower weights for voxels positioned more distal to the mid-thickness surface.
Additionally, we developed a Python-based framework for network-based analyses of the vasculature’s structure. Skeleton graph XML files are parsed to define segment type (start, termination, branchpoint, or skeleton) by examining the degree of connectivity, as well as connecting edges using the NetworkX package (110). Each edge represents a physical connection between two nodes of type i) start–skeleton, ii) bifurcation–skeleton, iii) skeleton–skeleton, or iv) skeleton-end with properties defining length, diameter, volume, and surface area. Nodes and edges are used to construct a network for extraction of the shortest path from a given node to the rest of the vascular tree, as well as to compute different network characteristics (e.g., connected components, lowest common ancestors). Finally, vascular networks can be visualized and inspected interactively using implementation of the plotly interface. Individual MeVisLab workflows, output files, and visualization notebooks for each subject and hemisphere can be found in the online repository.
Hippocampal BigBrain Data.
Additional vertex-wise metrics provided by DeKraker et al. (36), based on the BigBrain’s hippocampal i) morphometry and ii) cell body staining intensity were used to correlate with the perfusion data. Briefly, the morphometry-based features relate to different aspects of the hippocampus’ shape—thickness, curvature, gyrification, and inner and outer texture—and were constructed after unfolding using its manual segmentation. In addition, the changes in staining intensity along the hippocampal inner and outer GM boundaries were quantified on a per vertex-basis using their intensity profile features based on mean X, mean Y, SD X, skewness and kurtosis (Fig. 4C). Their differences indicate differences in cell body density properties. For example, more cell body dense regions will be characterized by a higher mean Y than less dense regions.
Perfusion Data Reliability Analyses.
The low microvascular density poses challenges for ASL-based perfusion data (27), resulting in relatively low SNR, especially at the required spatial resolutions for hippocampal subfield imaging. To study stability and sensitivity in detecting intra-hippocampal differences and to address potential sampling biases, we constructed N = 1,000 hippocampal perfusion maps by aggregating data from multiple runs and subjects, shuffling the order of subjects and runs each iteration, and calculating the median value. It is important to note that the left and right hemisphere data were averaged for each iteration as they were acquired simultaneously during a single run. Alongside the mean and variability of perfusion estimates, we assessed the dependence of perfusion tSNR and the effect size of between-subfield differences using Friedman’s test Q-statistic (Statistical Analyses).
Statistical Analyses.
Statistical analyses were performed using the pingouin Python package (111). The non-parametric Friedman’s test for repeated measures analyses of variance was used to assess differences across subfields. In case of a significant subfield effect, the Wilcoxon signed rank-test was applied for pairwise-comparisons, correcting for multiple comparison using the Benjamini–Hochberg false-discovery rate (FDRBH) method. The Pearson’s correlation coefficient was used to assess correlations among hippocampal surface maps (e.g., perfusion vs. T1w/T2w), while controlling for spatial autocorrelation (112) using “roll”-based permutation testing (proll) as well as multiple comparisons using FDRBH correction when constructing the correlation heatmaps. Briefly, to generate null distributions, N = 5000 permuted maps are generated by randomly shifting the 2D hippocampal maps across one or both axes using SciPy’s shift function and through rotation using their rotate function (113). Here, extension of maps was ensured by wrapping around to the opposite edge. Significance was then determined based on the position of the empirical correlation coefficient with respect to the generated null distribution (6).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to thank the participants who agreed to take part in this study. Author A.R.K received support by the Canada Foundation for Innovation John R. Evans Leaders Fund project (grant agreement no. 37427), a Canadian Institutes of Health Research Project Grant (366062), and Canada Research Chairs (950-231964). Author B.P. has received partial funding from the Dutch Research Council under grant VIDI-TTW (016-178-052), European Union’s Horizon 2020 research and innovation program (885876) and NIH (R01MH111444). Authors R.A.M.H and J.K. were supported by BrainsCAN and Natural Sciences and Engineering Research Council of Canada postdoctoral fellowships, respectively.
Author contributions
R.A.M.H. and S.K. designed research; R.A.M.H., S.K., and M.D.Y. performed research; R.A.M.H., S.K., D.I., B.A.P., J.D. and A.R.K. contributed new reagents/analytic tools; R.A.M.H. and S.K. analyzed data; and R.A.M.H., S.K., D.I., J.D., B.A.P., and A.R.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Roy A. M. Haast, Email: rhaast@uwo.ca.
Sriranga Kashyap, Email: sriranga.kashyap@uhn.ca.
Data, Materials, and Software Availability
Anonymized (MRI-derived data & code) data have been deposited in https://github.com/royhaast/hippocampal_perfusion (114).
Supporting Information
References
- 1.Eichenbaum H., Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron 44, 109–120 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Squire L. R., Stark C. E. L., Clark R. E., The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Ding S.-L., Van Hoesen G. W., Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemoarchitecture. J. Comp. Neurol. 523, 2233–2253 (2015). [DOI] [PubMed] [Google Scholar]
- 4.DeKraker J., Köhler S., Khan A. R., Surface-based hippocampal subfield segmentation. Trends Neurosci. 44, 856–863 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Genon S., Bernhardt B. C., La Joie R., Amunts K., Eickhoff S. B., The many dimensions of human hippocampal organization and (dys) function. Trends Neurosci. 44, 977–989 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karat B. G., DeKraker J., Hussain U., Köhler S., Khan A. R., Mapping the macrostructure and microstructure of the in vivo human hippocampus using diffusion MRI. Hum. Brain Mapp. 44, 5485–5503 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel R., et al. , Investigating microstructural variation in the human hippocampus using non-negative matrix factorization. Neuroimage 207, 116348 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Maass A., et al. , Laminar activity in the hippocampus and entorhinal cortex related to novelty and episodic encoding. Nat. Commun. 5, 5547 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aitken F., Kok P., Hippocampal representations switch from errors to predictions during acquisition of predictive associations. Nat. Commun. 13, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bussy A., et al. , Hippocampal shape across the healthy lifespan and its relationship with cognition. Neurobiol. Aging 106, 153–168 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Wisse L. E. M., et al. , Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normal aging. Neurobiol. Aging 35, 2039–2045 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wolf D., Fischer F. U., de Flores R., Chételat G., Fellgiebel A., Differential associations of age with volume and microstructure of hippocampal subfields in healthy older adults. Hum. Brain Mapp. 36, 3819–3831 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radhakrishnan H., Stark S. M., Stark C. E. L., Microstructural alterations in hippocampal subfields mediate age-related memory decline in humans. Front. Aging Neurosci. 12, 94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small S. A., Schobel S. A., Buxton R. B., Witter M. P., Barnes C. A., A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 12, 585–601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blümcke I., et al. , International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on diagnostic methods. Epilepsia 54, 1315–1329 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Cembrowski M. S., Wang L., Sugino K., Shields B. C., Spruston N., Hipposeq: A comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife 5, e14997 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X., et al. , Transcriptional profiling reveals strict boundaries between hippocampal subregions. J. Comp. Neurol. 441, 187–196 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Alkadhi K. A., Cellular and molecular differences between area ca1 and the dentate gyrus of the hippocampus. Mol. Neurobiol. 56, 6566–6580 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Podgorny O. V., Gulyaeva N. V., Glucocorticoid-mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis. J. Neurochem. 157, 370–392 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Hsu J. C., et al. , Decreased expression and functionality of NMDA receptor complexes persist in the CA1, but not in the dentate gyrus after transient cerebral ischemia. J. Cereb. Blood Flow Metab. 18, 768–775 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Duvernoy H. M., Cattin F., Risold P.-Y., The Human Hippocampus: Functional Anatomy. Vascularization and Serial Sections with MRI (Springer-Verlag, Berlin/Heidelberg, Germany, ed. 4, 2013). [Google Scholar]
- 22.Cembrowski M. S., Spruston N., Heterogeneity within classical cell types is the rule: Lessons from hippocampal pyramidal neurons. Nat. Rev. Neurosci. 20, 193–204 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Perosa V., et al. , Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain 143, 622–634 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spallazzi M., et al. , Hippocampal vascularization patterns: A high-resolution 7 Tesla time-of-flight magnetic resonance angiography study. Neuroimage: Clinical 21, 101609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buch S., Chen Y., Jella P., Ge Y., Mark Haacke E., Vascular mapping of the human hippocampus using Ferumoxytol-enhanced MRI. Neuroimage 250, 118957 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Detre J. A., Leigh J. S., Williams D. S., Koretsky A. P., Perfusion imaging. Magn. Reson. Med. 23, 37–45 (1992). [DOI] [PubMed] [Google Scholar]
- 27.Uludağ K., Blinder P., Linking brain vascular physiology to hemodynamic response in ultra-high field MRI. Neuroimage 168, 279–295 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Chang C., et al. , The bumps under the hippocampus. Hum. Brain Mapp. 39, 472–490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L. E. M. Wisse et al., A harmonized segmentation protocol for hippocampal and parahippocampal subregions: Why do we need one and what are the key goals? Hippocampus 27, 3–11 (2017). [DOI] [PMC free article] [PubMed]
- 30.Gross D. W., Misaghi E., Steve T. A., Wilman A. H., Beaulieu C., Curved multiplanar reformatting provides improved visualization of hippocampal anatomy. Hippocampus 30, 156–161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov D., Poser B. A., Huber L., Pfeuffer J., Uludağ K., Optimization of simultaneous multislice epi for concurrent functional perfusion and bold signal measurements at 7T. Magn. Reson. Med. 78, 121–129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohmann R., Speck O., Scheffler K., Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 Tesla using current receive coil arrays. Magn. Reson. Med. 75, 801–809 (2016). [DOI] [PubMed] [Google Scholar]
- 33.DeKraker J., et al. , Automated hippocampal unfolding for morphometry and subfield segmentation with Hippunfold. eLife 11, e77945 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeKraker J., Lau J. C., Ferko K. M., Khan A. R., Köhler S., Hippocampal subfields revealed through unfolding and unsupervised clustering of laminar and morphological features in 3D BigBrain. Neuroimage 206, 116328 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Amunts K., et al. , BigBrain: An ultrahigh-resolution 3D human brain model. Science 340, 1472–1475 (2013). [DOI] [PubMed] [Google Scholar]
- 36.DeKraker J., Ferko K. M., Lau J. C., Köhler S., Khan A. R., Unfolding the hippocampus: An intrinsic coordinate system for subfield segmentations and quantitative mapping. Neuroimage 167, 408–418 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Witter M. P., et al. , Organization of the entorhinal-hippocampal system: A review of current anatomical data. Hippocampus 3, 33 (1993). [PubMed] [Google Scholar]
- 38.Parkes L. M., Rashid W., Chard D. T., Tofts P. S., Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn. Reson. Med. 51, 736–743 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Kashyap S., et al. , The impact of b1+ on the optimisation of high-resolution ASL acquisitions at 7T. Proc. Intl. Soc. Mag. Reson. Med. 29, 1417 (2021). [Google Scholar]
- 40.Kashyap S., Ivanov D., Havlicek M., Poser B. A., Uludağ K., Impact of acquisition and analysis strategies on cortical depth-dependent fMRI. Neuroimage 168, 332–344 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Andersson J. L. R., Skare S., Ashburner J., How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Alsop D. C., et al. , Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donahue M. J., Lu H., Jones C. K., Pekar J. J., van Zijl P. C. M., An account of the discrepancy between MRI and pet cerebral blood flow measures. A high-field MRI investigation. NMR Biomed. 19, 1043–1054 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Shaw K., et al. , Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat. Commun. 12, 3190 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair V., Palm D., Roth L. J., Relative vascularity of certain anatomical areas of the brain and other organs of the rat. Nature 188, 497–498 (1960). [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., et al. , High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice. Natl. Sci. Rev. 6, 1223–1238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavaglia M., et al. , Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 910, 81–93 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Soltesz I., Losonczy A., Ca1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat. Neurosci. 21, 484–493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.E. K. Michaelis, “Selective neuronal vulnerability in the hippocampus: Relationship to neurological diseases and mechanisms for differential sensitivity of neurons to stress” in The Clinical Neurobiology of the Hippocampus: An Integrative View, T. Bartsch, Ed. (Oxford University Press, 2012).
- 50.Pfeuffer J., et al. , Perfusion-based high-resolution functional imaging in the human brain at 7 Tesla. Magn. Reson. Med. 47, 903–911 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Duvernoy H. M., Cattin F., Risold P.-Y., The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI (Springer Science & Business Media, 2013). [Google Scholar]
- 52.Chappell M. A., et al. , Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn. Reson. Med. 65, 1173–1183 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Atilla Erdem M., Yaşargil G., Roth P., Microsurgical anatomy of the hippocampal arteries. J. Neurosurg. 79, 256–265 (1993). [DOI] [PubMed] [Google Scholar]
- 54.Wisse L. E. M., et al. , Automated hippocampal subfield segmentation at 7T MRI. Am. J. Neuroradiol. 37, 1050–1057 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivanov D., et al. , Comparison of 3T and 7T ASL techniques for concurrent functional perfusion and bold studies. Neuroimage 156, 363–376 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Marques J. P., et al. , Mp2rage, a self bias-field corrected sequence for improved segmentation and t1-mapping at high field. Neuroimage 49, 1271–1281 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Eggenschwiler F., Kober T., Magill A. W., Gruetter R., Marques J. P., Sa2rage: A new sequence for fast b1+-mapping Magn. Reson. Med. 67, 1609–1619 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Marques J. P., Gruetter R., New developments and applications of the mp2rage sequence-focusing the contrast and high spatial resolution r1 mapping. PLoS ONE 8, e69294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haast R. A. M., et al. , Effects of mp2rage b1+ sensitivity on inter-site t1 reproducibility and hippocampal morphometry at 7T. Neuroimage 224, 117373 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Chappell M. A., Groves A. R., Whitcher B., Woolrich M. W., Variational Bayesian inference for a nonlinear forward model. IEEE Trans. Signal Process. 57, 223–236 (2008). [Google Scholar]
- 61.Olsen R. K., et al. , Progress update from the hippocampal subfields group. Alzheimer’s Dementia 11, 439–449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teeuwisse W. M., Webb A. G., van Osch M. J. P., Arterial spin labeling at ultra-high field: All that glitters is not gold. Int. J. Imaging Syst. Technol. 20, 62–70 (2010). [Google Scholar]
- 63.Teeuwisse W. M., Brink W. M., Webb A. G., Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magn. Reson. Med. 67, 1285–1293 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Hurley A. C., et al. , Tailored RF pulse for magnetization inversion at ultrahigh field. Magn. Reson. Med. 63, 51–58 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Petr J., et al. , Effects of systematic partial volume errors on the estimation of gray matter cerebral blood flow with arterial spin labeling MRI. Magn. Reson. Mater. Phys., Biol. Med. 31, 725–734 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Günther M., Oshio K., Feinberg D. A., Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn. Reson. Med. 54, 491–498 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Vidorreta M., et al. , Evaluation of segmented 3D acquisition schemes for whole-brain high-resolution arterial spin labeling at 3T. NMR Biomed. 27, 1387–1396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D. Kurban et al., “High-resolution perfusion and blood-volume fMRI at 7T with simultaneous multi-slice spiralout acquisitions” in Proceedings of the 37th Annual Scientific Meeting, ESMRMB Online (2020).
- 69.Kashyap S., et al. , Sub-millimetre resolution laminar fMRI using arterial spin labelling in humans at 7T. PLoS ONE 16, e0250504 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woods J. G., Chappell M. A., Okell T. W., A general framework for optimizing arterial spin labeling MRI experiments. Magn. Reson. Med. 81, 2474–2488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan A. P., et al. , Long-delay arterial spin labeling provides more accurate cerebral blood flow measurements in Moyamoya patients. Stroke 48, 2441–2449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang N., Gordon M. L., Goldberg T. E., Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci. Biobehav. Rev. 72, 168–175 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Guo X., et al. , Asymmetry of cerebral blood flow measured with three-dimensional pseudocontinuous arterial spin-labeling MR imaging in temporal lobe epilepsy with and without mesial temporal sclerosis. J. Magn. Reson. Imaging 42, 1386–1397 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Lieberman J. A., et al. , Hippocampal dysfunction in the pathophysiology of schizophrenia: A selective review and hypothesis for early detection and intervention. Mol. Psychiatry 23, 1764–1772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Opheim G., et al. , 7T epilepsy task force consensus recommendations on the use of 7T MRI in clinical practice. Neurology 96, 327–341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaitsev M., Maclaren J., Herbst M., Motion artifacts in MRI: A complex problem with many partial solutions. J. Magn. Reson. Imaging 42, 887–901 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zun Z., Shankaranarayanan A., Zaharchuk G., Pseudocontinuous arterial spin labeling with prospective motion correction (PCASL-PROMO). Magn. Reson. Med. 72, 1049–1056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Power J. D., et al. , Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.S. Kashyap, Í. A. F. Oliveira, K. Uludağ, Feasibility of high-resolution perfusion imaging using arterial spin labelling MRI at 3 Tesla. Front. Physiol. 14, 1271254 (2024). [DOI] [PMC free article] [PubMed]
- 80.Johnson A. C., Hippocampal vascular supply and its role in vascular cognitive impairment. Stroke 54, 673–685 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bollmann S., et al. , Imaging of the pial arterial vasculature of the human brain in vivo using high-resolution 7T time-of-flight angiography. eLife 11, e71186 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haines K., Smith N. B., Webb A. G., New high dielectric constant materials for tailoring the b1+ distribution at high magnetic fields J. Magn. Reson. 203, 323–327 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Haast R. A. M., Ivanov D., Uludağ K., The impact of correction on mp2rage cortical t 1 and apparent cortical thickness at 7T. Hum. Brain Mapp. 39, 2412–2425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hennig J., Nauerth A., Friedburg H., Rare imaging: A fast imaging method for clinical MR. Magn. Reson. Med. 3, 823–833 (1986). [DOI] [PubMed] [Google Scholar]
- 85.Axel L., Blood flow effects in magnetic resonance imaging. Magn. Reson. Annu. 1986, 237–244 (1986). [PubMed] [Google Scholar]
- 86.Parker D. L., Yuan C., Blatter D. D., MR angiography by multiple thin slab 3D acquisition. Magn. Reson. Med. 17, 434–451 (1991). [DOI] [PubMed] [Google Scholar]
- 87.Wehrli F. W., Time-of-flight effects in MR imaging of flow. Magn. Reson. Med. 14, 187–193 (1990). [DOI] [PubMed] [Google Scholar]
- 88.Kwong K. K., et al. , MR perfusion studies with t1-weighted echo planar imaging. Magn. Reson. Med. 34, 878–887 (1995). [DOI] [PubMed] [Google Scholar]
- 89.Kim S.-G., Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (fair) technique: Application to functional mapping. Magn. Reson. Med. 34, 293–301 (1995). [DOI] [PubMed] [Google Scholar]
- 90.Wong E. C., Buxton R. B., Frank L. R., Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn. Reson. Med. 39, 702–708 (1998). [DOI] [PubMed] [Google Scholar]
- 91.S. Kashyap, srikash/presurfer: ondu, 2021. GitHub. https://github.com/srikash/presurfer. Accessed 22 February 2021.
- 92.Dale A. M., Fischl B., Sereno M. I., Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999). [DOI] [PubMed] [Google Scholar]
- 93.Yushkevich P. A., et al. , Fast automatic segmentation of hippocampal subfields and medial temporal lobe subregions in 3 Tesla and 7 Tesla t2-weighted MRI. Alzheimer’s Dementia 7, P126–P127 (2016). [Google Scholar]
- 94.Aguirre G. K., Detre J. A., Zarahn E., Alsop D. C., Experimental design and the relative sensitivity of bold and perfusion fMRI. Neuroimage 15, 488–500 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Liu T. T., Wong E. C., A signal processing model for arterial spin labeling functional MRI. Neuroimage 24, 207–215 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Buxton R. B., et al. , A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 40, 383–396 (1998). [DOI] [PubMed] [Google Scholar]
- 97.Chappell M. A., et al. , A toolbox for perfusion quantification using arterial spin labelling. Imaging Neurosci. 1, 1–16 (2023). [Google Scholar]
- 98.Rane S. D., Gore J. C., Measurement of t1 of human arterial and venous blood at 7T. Magn. Reson. Imaging 31, 477–479 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanchez R. M., Panchuelo O. M., Turner R., Francis S. T., Quantitative t1 mapping using multi-slice multi-shot inversion recovery EPI. Neuroimage 234, 117976 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yushkevich P. A., et al. , User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Köster J., Rahmann S., Snakemake—A scalable bioinformatics workflow engine. Bioinformatics 28, 2520–2522 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Marcus D., et al. , Informatics and data mining tools and strategies for the human connectome project. Front. Neuroinform. 5, 4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerig G., Kubler O., Kikinis R., Jolesz F. A., Nonlinear anisotropic filtering of MRI data. IEEE Trans. Med. Imaging 11, 221–232 (1992). [DOI] [PubMed] [Google Scholar]
- 104.Weickert J., Coherence-enhancing diffusion filtering. Int. J. Comput. Vision 31, 111–127 (1999). [Google Scholar]
- 105.Gulban O. F., Schneider M., Marquardt I., Haast R. A. M., De Martino F., A scalable method to improve gray matter segmentation at ultra high field MRI. PLoS ONE 13, e0198335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ritter F., et al. , Medical image analysis. IEEE Pulse 2, 60–70 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Selle D., Preim B., Schenk A., Peitgen H.-O., Analysis of vasculature for liver surgical planning. IEEE Trans. Med. Imaging 21, 1344–1357 (2002). [DOI] [PubMed] [Google Scholar]
- 108.Mattern H., Book of Abstracts ESMRMB 2021 Online 38th Annual Scientific Meeting 7–9 October 2021. MAGMA 34, 1–204 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.M. Brett et al., nipy/nibabel: 2.4.1. Zenodo. 10.5281/zenodo.3233118. Accessed 24 October 2021. [DOI]
- 110.A. Hagberg, P. Swart, D. S. Chult. “Exploring network structure, dynamics, and function using networkx” (Tech. Rep., Los Alamos National Lab (LANL), Los Alamos, NM, 2008).
- 111.Vallat R., Pingouin: Statistics in Python. J. Open Source Software 3, 1026 (2018). [Google Scholar]
- 112.Markello R. D., Misic B., Comparing spatial null models for brain maps. Neuroimage 236, 118052 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Virtanen P., et al. , SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 17, 261–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.R. A. M. Haast, S. Kashyap, royhaast/hippocampal_perfusion: manuscript-acceptance (v1.0.0). Zenodo. 10.5281/zenodo.10644517. Deposited 16 June 2023. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Anonymized (MRI-derived data & code) data have been deposited in https://github.com/royhaast/hippocampal_perfusion (114).