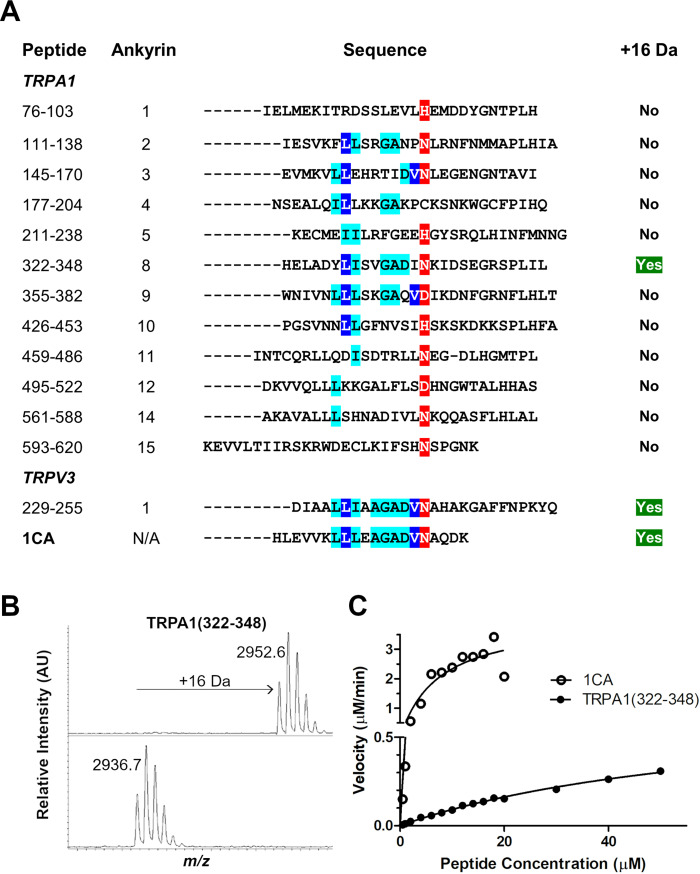
Figure 1.
FIH catalyses the hydroxylation of ankyrin repeat domain fragments from TRPA1 and TRPV3. A) Alignment of TRPA1‐ and TRPV3 (residues 229–255)‐derived peptides with the 1CA consensus ARD sequence. [28] B) Matrix‐assisted laser desorption (MALDI) MS spectra showing a +16 Da mass shift of 10 μM TRPA1 (322–348), as catalysed by FIH. Conditions: FIH (2 μM, top panel; 0 μM, bottom panel), sodium ascorbate (100 μM), 2‐OG (100 μM), ferrous ammonium sulfate (20 μM) in 50 mM Tris buffer (pH 7.5, 37 °C, 1 h). C) Comparison of hydroxylation of peptides by FIH as assessed by SPE‐MS. Conditions: FIH (0.1 μM, 1CA; 0.4 μM, TRPA1 (322–348)), sodium ascorbate (100 μM), 2‐OG (100 μM), ferrous ammonium sulfate (20 μM) in 50 mM Tris buffer (pH 7.5, RT). Error bars represent SEM for n=2 experiments performed in triplicate.