ABSTRACT
Background
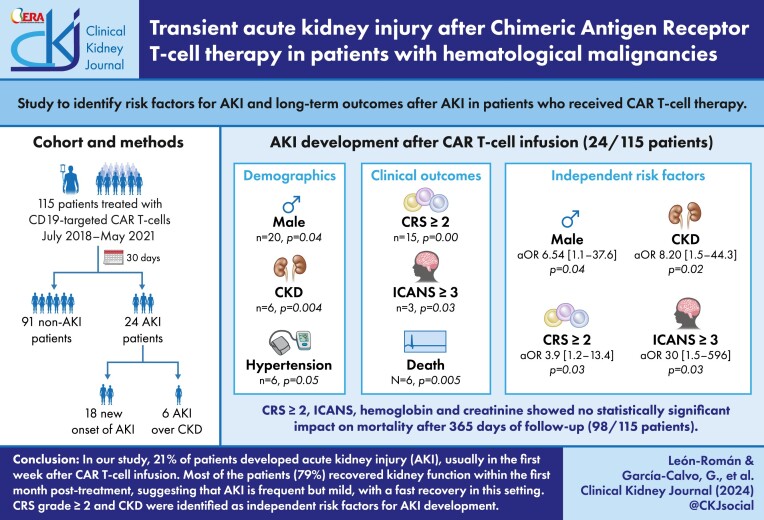
Acute kidney injury (AKI) occurs in 30% of patients infused with chimeric antigen receptor (CAR) T-cells. The purpose of this study was to identify risk factors and long-term outcomes after AKI in patients who received CAR T-cell therapy.
Methods
Medical records of 115 adult patients with R/R hematological malignancies treated with CD19-targeted CAR T-cells at Vall d'Hebron University Hospital between July 2018 and May 2021. Baseline demographic data including age, gender, ethnicity, body mass index (BMI), and co-morbidities, as well as the type of hematological neoplasia and prior lines of therapy were collected. Laboratory parameters including serum creatinine and whole blood hemoglobin were retrospectively reviewed and values were gathered for days +1, +7, +14, +21, and +28 post-infusion.
Results
A total of 24/115 (21%) patients developed AKI related to CAR T-cell therapy; 6/24 with AKI over chronic kidney disease (CKD). Two patients had AKI in the context of lymphodepleting (LD) chemotherapy and the other 22 after CAR T-cell infusion, starting at day+1 in 3 patients, day+7 in 13 patients, day +14 in 1 patient, day+21 in 2 patients, and day+28 in 3 patients. Renal function was recovered in 19/24 (79%) patients within the first month after infusion. Male gender, CKD, cytokine release syndrome (CRS), and immune effector cell-associated neurotoxicity syndrome (ICANS) were associated with AKI. Male gender, CKD, ICANS grade ≥3 and CRS grade ≥2 were identified as independent risk factors for AKI on multivariable analysis. In terms of the most frequent CAR T-cell related complications, CRS was observed in 95 (82%) patients and ICANS in 33 (29%) patients. Steroids were required in 34 (30%) patients and tocilizumab in 37 (32%) patients. Six (5%) patients were admitted to the intensive care unit (1 for septic shock, 4 for CRS grade ≥2 associated to ICANS grade ≥2, and 1 for CRS grade ≥3). A total of 5 (4.4%) patients died in the first 30 days after CAR T-cell infusion for reasons other than disease progression, including 4 cases of infectious complications and 1 of heart failure.
Conclusion
Our results suggest that AKI is a frequent but mild adverse event, with fast recovery in most patients.
Keywords: acute kidney injury, CAR-T therapy, onconephrology
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
CAR T-cell therapy has increased survival of patients with relapsed/refractory hematological malignancies.
Acute kidney injury (AKI) occurs in 20–30% of patients receiving CAR T-cell therapy.
Risk factors for acute kidney injury development in the setting of CAR T-cell therapy are not completely established.
This study adds:
Clinical characteristics of AKI in one of the largest series of hematological cancer patients treated with CAR T-cell therapy.
We demonstrated that AKI is mainly a transient complication in patients treated with CAR T-cell therapy.
In our cohort, males, CKD, ICANS, and CRS were identified as risk factors for AKI after CAR T-cell therapy.
Potential impact:
Preventing and diagnosing AKI in patients treated with CAR T-cells.
Strict monitoring of renal function in patients at risk, mainly male patients, previous history of CKD, and those developing CRS and ICANS after infusion.
Early Nephrology referral in patients treated with CAR T-cells who develop AKI without a fast renal function recovery.
INTRODUCTION
Chimeric antigen receptors (CARs) are engineered synthetic proteins that redirect the specificity of T cells [1, 2]. The structure of commercially available CARs includes extracellular immunoglobulin-derived heavy and light chains to recognize specific antigens, and intracellular activating and costimulatory domains to lead signal activation, cytokine release, T-cell proliferation, and immune cell response against tumor cells. [1, 3–5]
CAR T-cell therapy has been a revolutionary treatment for relapsed/refractory (R/R) hematological malignancies including adult and pediatric B-cell acute lymphoblastic leukemia, diffuse large B-cell lymphoma, follicular lymphoma and multiple myeloma [6–21]. Furthermore, there are promising results in selected solid tumors and autoimmune diseases [22, 23]. Response rates and long-term survival are variable across diseases but they outweigh the current available therapies in these indications.
CAR T-cell therapy has a well-known acute and long-term toxicity profile [24, 25]. The most frequent acute adverse event is cytokine release syndrome (CRS), occurring usually within the first week after CAR T-cell infusion [7–20, 22–30]. CRS manifests as a rapid immune reaction driven by the massive release of cytokines, including IFN-gamma and IL-6 [29–31]. The clinical presentation includes fever, hypotension, and/or hypoxia. Organ dysfunction can sometimes occur in this setting but does not impact CRS grading [29–32]. Immune effector cell-associated neurotoxicity syndrome (ICANS) is another frequent acute side effect which usually starts around the second week post-infusion, after CRS onset in the great majority of patients. Acute kidney injury (AKI) develops in 20–30% of infused patients, mainly associated with CRS, electrolyte disorders and tumor lysis syndrome (TLS) [33–35]. Regarding late complications, after the first month post-infusion, the most common are hypogammaglobulinemia, cytopenia, and infections [24–28, 29].
There is limited literature regarding AKI after CAR-T cell therapy. Kanduri et al. described in a systematic review of 22 cohort studies, a population of 3376 pediatric and adult patients that included the incidence of AKI and clinical complications after CAR T-cell infusion. The pooled estimated incidence of AKI was 18.6%, and AKI was presented in 17% in the subgroup of adults after the treatment. The estimated CRS incidence in all included studies was 75.4% [36]. The purpose of this study is to determine the demographics, laboratory results and clinical evolution of patients who received CAR T-cell therapy and developed AKI, as well as identifying potential risk factors.
MATERIALS AND METHODS
Data collection and analysis
We conducted a retrospective review of the medical records of 115 adult patients with R/R hematological malignancies treated with CD19-targeted CAR T-cells at Vall d'Hebron University Hospital between July 2018 and May 2021 (Fig. 1). Data collection was performed with the approval of the Ethics Committee of the Vall d'Hebron University Hospital EOM(AG)043/2021(5853).
Figure 1:

Flow diagram for study selection.
Baseline demographic data including age, gender, ethnicity, body mass index (BMI), and co-morbidities, as well as the type of hematological neoplasia and prior lines of therapy were collected. Laboratory parameters including serum creatinine and whole blood hemoglobin were retrospectively reviewed and values were gathered for days +1, +7, +14, +21, and +28 post-infusion.
Definition and endpoints
AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria: grade 1, increase in serum creatinine (SCr) 1.5 to <2-fold of baseline; grade 2, increase in SCr 2- to <3-fold of baseline; grade 3, increase in SCr ≥3-fold of baseline, OR requiring renal replacement therapy, OR SCr ≥4.0 mg/dL [37]. Chronic kidney disease (CKD) was defined according KDIGO as kidney damage or glomerular filtration rate (GFR) <60 ml/min/1.73 m2 with CKD-EPI formula for 3 months or more, irrespective of cause [38]. Recovery of renal function was established following the consensus report of the Acute Disease Quality Initiative (ADQI) [39]. Severe electrolyte disorders were defined according to the Common Terminology Criteria for Adverse Events (CTCAE) [40].
CRS and ICANS were graded according to the American Society of Transplantation and Cellular Therapy (ASTCT) criteria. Other treatment-related adverse events such as infections intensive care unit (ICU) admission and death were also recorded [41, 42].
Lymphodepleting chemotherapy
All patients received LD chemotherapy on the week before CAR T-cell infusion. LD chemotherapy included cyclophosphamide and fludarabine for 3–4 consecutive days, according to the label recommendations for each disease.
Fludarabine required dose adjustment in case of a decreased creatinine clearance (CrCl), according to institutional guidelines. In brief: (i) if CrCl ≥70 mL/min/1.73 m2, no dose reduction was applied; (ii) if CrCl was 45–70 mL/min/1.73 m2, each daily dose was reduced by 25%; and (iii) if CrCl was 30–45 mL/min/1.73 m2, each daily dose was reduced by 40%.
Statistical analysis
First, patients were divided into two groups: (i) patients who developed AKI and (ii) patients without AKI. The distribution was calculated with the Kolmogorov Smirnov test. Quantitative variables were analysed with the Student's t test and presented with their mean and standard deviation or median and interquartile range. Qualitative variables were analysed with Chi-squared test or Fisher's exact coefficient, and presented with their frequency distribution. Bivariate and multivariate analyses were obtained with logistic regression to identify risk factors for AKI after CAR T-cell therapy. As a second step, actuarial survival curves were estimated using the Kaplan–Meier method to identify risk factors for mortality in a 365-day follow-up. Statistical studies were analysed using the SPSS version 20 program. Odds ratio (OR) for AKI with 95% confidence interval (CI) was reported. Two-sided P values 0.05 were considered statistically significant.
RESULTS
Patient characteristics
Median age was 61 years [range, 20–81] and 66% were male. Demographic and clinical characteristics for all patients are shown in Table 1. Briefly, hypertension was present in 37% of patients, diabetes in 8%, and cardiovascular disease in 5%. CKD was present in 11 (9%) patients due to obstructive nephropathy related to a retroperitoneal mass (5), unknown kidney disease (5) or chemotherapy-related toxicity (1). Hematological neoplasms included diffuse large B-cell lymphoma (91%), B-cell acute lymphoblastic leukemia (5%), mantle cell lymphoma (4%), and primary mediastinal large B-cell lymphoma (1%). The median of previous treatment lines was 2 [IQR, 1–3], 29 (25%) patients had undergone an autologous hematopoietic cell transplant (HCT), and 1 (1%) patient an allogeneic HCT. Fourteen (12%) patients required fludarabine dose adjustment for a decreased glomerular filtrate (<60 ml/min/1.73 m2 in 11 patients and <70 ml/min/1.73 m2 by Cockcroft–Gault formula in three patients). Regarding the type of CAR-T construct, 50% of patients received tisagenlecleucel, 28% lisocabtagene maraleucel, 20% axicabtagene ciloleucel, and 2% brexucabtagene autoleucel.
Table 1:
Demographic characteristics of the full patient population.
| Variables | All | AKI patients (n=24) | Non-AKI patients (n=91) | P value |
|---|---|---|---|---|
| Age, median [range] | 61 [20-81] | 16 (66.7) | 46 (50.5) | 0.16 |
| Males, n (%) | 76 (66) | 20 (83.3) | 56 (61.5) | 0.04 |
| Hypertension, n (%) | 43 (37.4) | 13 (54.2) | 30 (33.0) | 0.05 |
| Type 2 diabetes mellitus, n (%) | 9 (7.8) | 3 (12.5) | 6 (6.6) | 0.34 |
| Chronic kidney disease, n (%) | 11 (9.5) | 6 (25.0) | 5 (5.5) | 0.004 |
| Dose reduced fludarabine, n (%) | 14 (12.2) | 8 (33.3) | 6 (6.6) | 0.002 |
| CAR T-cell construct, n (%) | 0.04 | |||
| Lisocabtagene maraleucel | 32 (27.8) | 7 (29.2) | 25 (27.5) | |
| Tisagenlecleucel | 57 (49.6) | 14 (58.3) | 43 (47.3) | |
| Axicabtagene ciloleucel | 23 (20.0) | 1 (4.2) | 22 (24.2) | |
| Brexucabtagene autoleucel | 3 (2.6) | 2 (8.3) | 1 (1.1) |
AKI after CAR-T cell therapy
A total of 24 (21%) patients developed AKI after CAR T-cell therapy. Onset of decreased renal clearance took place during LD chemotherapy (with fludarabine and cyclophosphamide) in two patients and after CAR T-cell infusion in 22 patients. The latter group included three patients at day +1, 13 patients at day +7, one patient at day +14, two at day +21, and three patients at day +28. Among these cases, 17 (14.8%) had AKI stage 1, four (3.5%) had AKI stage 2, and three (2.6%) had AKI stage 3 (Table S1 and Table S2, see online supplementary material). Two patients required renal replacement therapy with a peak serum creatinine at day +7 of 2.45 and 2.0 mg/dL, respectively. One of whom died within one month from AKI stage 3 onset due to CRS grade 4 and refractory septic shock, and the last patient recovered kidney function within the first month after CAR-T cell therapy, achieving a serum creatinine of 0.4 mg/dL.
In terms of the AKI cohort, 19/24 (79%) patients recovered their baseline kidney function within the first month after CAR T-cell infusion [median 1 week, range 1–4 weeks]. The remaining five patients continued to present an impaired kidney function after two months of follow-up (4/5 diagnosed with new AKI onset) [median creatinine 1.4 mg/dL, range 1.3–2.4 mg/dL]. Regarding the CKD group, five out of the six patients who developed AKI recovered baseline kidney function within 30 days of CAR T-cell infusion, while the remaining patient developed a decreased creatinine clearance secondary to CRS grade 2 (from 34 ml/min/1.73 m2 to 27 ml/min1.73 m2) and maintained it after the 30-day of follow-up period. There were no significant differences in ICU admission and/or mortality in this CKD group.
Changes in serum electrolytes
In our cohort there were no cases of severe electrolyte disorders. The rate of hyponatremia (sodium <135 mmol/L) reached 15%, hypophosphatemia (phosphate <2.5 mg/dL) in 22% and hypocalcemia (calcium <8.5 mg/dL) in 43% by day +7, hypokalemia (potassium <3.5 mmol/L) in 21% and hypomagnesemia (magnesium <2 mg/dL) in 74% by day +21. In comparison between the AKI and non-AKI groups, hypophosphatemia and hypocalcemia by day +1 (P = 0.002 and P = 0.01, respectively), hyponatremia and hypocalcemia by day + 7 (P = 0.001 and P = 0.03, respectively), hypocalcemia by day +14 (P = 0.03), and hyponatremia by day +21 (P = 0.04) showed significant differences in the univariable analysis. However, these differences were not seen in the adjusted multivariable analysis.
CAR T-cell therapy complications and mortality
The most frequent CAR T-cell related adverse events were CRS (82% any grade and 30% CRS grade ≥2) and ICANS (29% any grade, 4% grade ≥3) (see Table 2). To manage these adverse events, 37 (32%) patients required tocilizumab and 34 (30%) patients received steroids. Six patients were admitted to the ICU (one with the diagnosis of refractory septic shock, four for CRS grade ≥2 associated to ICANS grade ≥2, and one for CRS grade ≥3). Median ICU stay was 8 days [range, 2–30 days]. Thirty-six patients died during study follow-up, 31 due to disease progression and five related to CAR-T cell therapy, including four cases of bacterial refractory septic shock and one case of heart failure.
Table 2:
Clinical outcomes after CAR T-cell infusion in the 115 patients.
| Variables | All | AKI patients (n=24) | Non-AKI patients (n=91) | P value |
|---|---|---|---|---|
| ICANS any grade, n (%) | 33 (28.7) | 11 (45.8) | 22 (24.2) | 0.04 |
| ICANS grade ≥3, n (%) | 4 (3.5) | 3 (12.5) | 1 (1.1) | 0.03 |
| CRS any grade, n (%) | 95 (81.9) | 21 (87.5) | 74 (81.3) | 0.56 |
| CRS grade ≥2, n (%) | 35 (30.2) | 15 (71.4) | 20 (27) | 0.001 |
| Steroid therapy, n (%) | 34 (29.6) | 19 (62.5) | 15 (20.9) | 0.001 |
| Tocilizumab, n (%) | 37 (32.2) | 16 (66.7) | 21 (23.1) | 0.001 |
| ICU admission, n (%) | 6 (5.2) | 3 (12.5) | 3 (3.3) | 0.07 |
| Death, n (%) | 0.005 | |||
| Due to disease progression | 31 (86.1) | 3 (50.0) | 28 (93.3) | |
| Non relapse related mortality | 5 (13.9) | 3 (50) | 2 (6.7) |
CRS: cytokine release syndrome; ICANS: immune effector cell-associated neurotoxicity syndrome; ICU: intensive care unit.
Risk factors for AKI and death
In the bivariate analysis, male gender (P = 0.04), previous history of CKD (P = 0.004), ICANS grade ≥3 (P = 0.03), CRS grade ≥2 (P = 0.001), and tocilizumab treatment (P = 0.001) were associated with AKI. In the multivariable analysis, male gender [adjusted odds ratio (aOR) 6.54, 95% CI 1.14–37.6, P = 0.04], CKD [aOR 8.20, 95% CI 1.52–44.3, P = 0.02], ICANS grade ≥3 [aOR 29.9, 95% CI 1.50-596.9, P = 0.03], and CRS grade ≥2 [aOR 3.98, 95% CI 1.18–13.4, P = 0.03] remained significant risk factors for AKI (Table 3). In the actuarial survival analysis one year after CAR T-cell infusion for 98/115 patients, CRS grade ≥2, ICANS any grade, hemoglobin levels and creatinine at day +1, +7, +28 showed no statistically significant impact on mortality (Fig. S2, see online supplementary material).
Table 3:
Adjusted odds ratio for AKI development.
| Variable | aOR | CI 95% | P value |
|---|---|---|---|
| Age ≥61 years | 2.24 | 0.66–7.65 | 0.20 |
| Males | 6.54 | 1.14–37.6 | 0.04 |
| CKD | 8.20 | 1.52–44.3 | 0.02 |
| ICANS grade≥3 | 29.9 | 1.50–596.9 | 0.03 |
| CRS grade≥2 | 3.98 | 1.18–13.4 | 0.03 |
aOR: adjusted odds ratio; CKD: chronic kidney disease; CRS: cytokine release syndrome; ICANS: immune effector cell-associated neurotoxicity syndrome.
Multivariate analysis was obtained with logistic regression.
Outcomes of patients with new AKI onset development and previous history of CKD
At a median follow-up of 296 days, the mean progression-free survival (PFS) was 198 days [95% CI, 100 to 297 days] in AKI patients vs 161 days [95% CI, 115 to 208 days] in non-AKI patients (P = 0.72). The mean overall survival (OS) was 281 days [95% CI, 27 to 227 days] in AKI patients versus 300 days [95% CI, 278 to 323 days] in non-AKI patients (P = 0.77).
The median PFS was 41 days [95% CI, 25 to 57 days] in CKD patients vs 132 days [95% CI, 81 to 182 days] in normal kidney function patients (P = 0.94). The OS was 305 days [95% CI, 230 to 380 days] in CKD patients versus 295 days [95% CI, 273 to 317 days] in normal kidney function patients (P = 0.33) (Fig. 2).
Figure 2:
Clinical outcomes in patients with AKI and previous history of CKD. (A) PFS and OS stratified by AKI development. (B) PFS and OS stratified by CKD.
DISCUSSION
CAR T-cell therapy has been a revolutionary treatment for R/R hematologic malignancies but it can lead to well-known severe complications. In our study, 24 (21%) patients developed AKI after CAR T-cell therapy, generally in the first week after infusion. Even though AKI is a complication of which treating physicians must be aware, it presents mostly with a mild disease and a rapid recovery, demonstrating that AKI is usually transient after CAR T-cell therapy. Our results are similar to those obtained by Gutgarts et al., who described AKI in 14 (30%) out of 46 patients with non-Hodgkin lymphoma receiving CAR T-cell therapy in the first 100 days after infusion. The most frequent presentation of AKI in this cohort was grade 1. In addition, 3 (21%) of the 14 AKI-patients died and 10 (71%) recovered baseline kidney function in a 30-day period [34]. Another study by Gupta et al. described 15/67 (22%) patients diagnosed with diffuse large B-cell lymphoma who developed AKI within 30 days of CAR T-cell infusion. The authors confirmed that AKI was usually a mild disease with fast recovery [35]. The largest study to date, led by Hanna et al., compared the risk of AKI in 232 patients who received CAR T-cell therapy with 414 patients who underwent auto-HCT, confirming no significant differences between both groups in the first 30 days after infusion [38]. These results are similar to our own findings, indicating that AKI associated with CAR T-cell infusion is a mild complication with a fast recovery.
Despite the lack of consensus on the optimal fludarabine dose reduction in patients with renal impairment, the incidence of AKI related to fludarabine exposure remains lower than 5% [44]. The need for dose adjustment in CKD patients relies on the 60% of drug excreted through urine [45]. Most studies suggest a 20–25% reduction for mild kidney impairment and up to a 50% reduction for moderate to severe impairment [46–48]. Wood et al. evaluated the outcomes of CAR T-cell therapy in patients with renal impairment and determined that progression-free survival and overall survival did not differ between patients with and without renal impairment or between those who received standard-dose fludarabine and those who received reduced-dose [49, 50]. Wood et al. also described that baseline renal function did not affect renal or efficacy outcomes after CAR T cell therapy. In contrast, in our study we found that patients with previous CKD had higher risk of AKI development, similarly to those described by Lyu et al. [50]. Our analysis also suggests that there were no differences in AKI development between standard and dose-reduced fludarabine after the multivariable analysis in accordance with Wood et al. [48].
Our study is the first that identifies male gender as an independent risk factor for AKI development. Furthermore, the most frequent complications after CAR T-cell infusion were CRS and ICANS. AKI was diagnosed in 88% of patients with CRS, and 46% in patients with ICANS. CRS grade ≥2 and ICANS grade ≥3 were adverse events identified as risk factors for AKI development. Similarly, other authors established CRS grade ≥3 as an independent risk factor for AKI [34, 35]. On the other hand, Ahmed et al. carried out a retrospective analysis of the impact of CKD and AKI on CAR-T outcomes in adult patients with non-Hodgkin’s lymphoma, observing that CKD patients had an increased frequency of CRS and ICANS [52]. Furthermore, Ahmed et al. defined ICANS ≥2 was an independent risk factor for AKI development [52]. We can surmise that proinflammatory status after CAR T-cell therapy may decrease renal perfusion and subsequently favor prerenal AKI development. Despite the risk of AKI with the proinflammatory state after CAR T-cell therapy, we consider that the potential benefit of the therapy warrants that clinicians actively monitor the development of CRS and/or ICANS.
Electrolyte disorders such as hypophosphatemia, hyponatremia, and hypokalemia are common after CAR T-cell therapy [34]. Farooqui et al. reported in their study 14/83 (17%) patients developing AKI within one month after CAR-T infusion. Moreover, they found that both absolute and relative from baseline to peak levels in lactate dehydrogenase were higher among AKI patients compared to non-AKI patients [53]. We observed that lower sodium, calcium, and phosphorus blood levels were associated with AKI in the univariable analysis. However, these findings were not confirmed in the multivariate analysis. This may be in part related to the lack of some laboratory assessments during the study follow-up.
Our study has some limitations, besides its retrospective design which precludes inferences of causal associations and selection bias. All patients included in the study received the CAR T-cell infusion, potentially overestimating the survival benefit of this treatment (immortal bias). Fludarabine dose adjustment was established according the Cockcroft–Gault formula even though we defined CKD based on the KDIGO definition (based on CKD-EPI formula). Blood test results could not be systematically compared as not all data were collected in our patient population. However, one of the strongest points of this study, in comparison with previously published articles, is that we had one of the largest cohorts and carried out long-term follow-up. Further multicenter studies with extended follow-up are needed to confirm our findings.
CONCLUSION
In our study, 21% of patients developed AKI, usually in the first week after CAR-T infusion. Most of the patients (79%) recovered kidney function within the first month post-treatment, suggesting that AKI is frequent but mild, with a fast recovery in this setting. Male gender and a history of CKD, together with development of CRS grade ≥2 and/or ICANS grade ≥3 after CAR T-cell infusion were identified as independent risk factors for AKI development.
Supplementary Material
ACKNOWLEDGEMENTS
J.L.-R. performed this work within the basis of his thesis at the Departamento de Medicina de la Universidad Autónoma de Barcelona.
Contributor Information
Juan León-Román, Nephrology Department, Vall d'Hebron University Hospital, Vall d'Hebron Institute of Research, CSUR National Unit of Expertise for Complex Glomerular Diseases of Spain, Barcelona, Spain.
Gloria Iacoboni, Department of Hematology, Vall d'Hebron University Hospital, Experimental Hematology, Vall d'Hebron Institute of Oncology (VHIO), Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron, Barcelona, Spain; Department of Medicine, Universitat Autònoma de Barcelona, Bellaterra, Spain.
Sheila Bermejo, N ephrology Department, Vall d'Hebron University Hospital, Vall d'Hebron Institute of Research, CSUR National Unit of Expertise for Complex Glomerular Diseases of Spain, Barcelona, Spain.
Cecilia Carpio, Department of Hematology, Vall d'Hebron University Hospital, Experimental Hematology, Vall d'Hebron Institute of Oncology (VHIO), Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron, Barcelona, Spain.
Mónica Bolufer, N ephrology Department, Vall d'Hebron University Hospital, Vall d'Hebron Institute of Research, CSUR National Unit of Expertise for Complex Glomerular Diseases of Spain, Barcelona, Spain.
Clara García-Carro, Nephrology Department, San Carlos Clinical University Hospital, Madrid, Spain.
Mario Sánchez-Salinas, Department of Hematology, Vall d'Hebron University Hospital, Experimental Hematology, Vall d'Hebron Institute of Oncology (VHIO), Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron, Barcelona, Spain.
Carla Alonso-Martínez, Pharmacy Department, Vall d´Hebron Hospital Universitari, Vall d´Hebron Barcelona Hospital Campus, Barcelona, Spain.
Oriol Bestard, N ephrology Department, Vall d'Hebron University Hospital, Vall d'Hebron Institute of Research, CSUR National Unit of Expertise for Complex Glomerular Diseases of Spain, Barcelona, Spain.
Pere Barba, Department of Hematology, Vall d'Hebron University Hospital, Experimental Hematology, Vall d'Hebron Institute of Oncology (VHIO), Vall d'Hebron Barcelona Hospital Campus, Passeig Vall d'Hebron, Barcelona, Spain.
María José Soler, N ephrology Department, Vall d'Hebron University Hospital, Vall d'Hebron Institute of Research, CSUR National Unit of Expertise for Complex Glomerular Diseases of Spain, Barcelona, Spain.
FUNDING
P.B. received research funding from the Carlos III Health Institute (PI21/0199,INT23/00072), Asociación Española contra el Cáncer (Ideas Semilla 2019) and a PERIS 2018-2020 grant from the Generalitat de Catalunya (BDNS357800). This research was funded by ISCIIII-FEDER and ISCIII RETICS REDinREN, grant number PI21/01292, ERA PerMed JTC2022 grant number AC22/00029, Río Hortega CM23/00213, Marató TV3 421/C/2020, Marató TV3 215/C/2021, and RICORS RD21/0005/0016. Enfermedad Glomerular Compleja del Sistema Nacional de Salud (CSUR), enfermedades glomerulares complejas.
AUTHORS’ CONTRIBUTIONS
J.L.-R. and M.J.S. collaborated on the original idea and study design. CG-C, S.B., C.C., M.B., C.G.-C., M.S.-S., C.A.-M., O.B., and P.B. contributed to the inclusion of patients. J.L.-R., GI, P.B., and M.J.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article itself.
CONFLICT OF INTEREST STATEMENT
M.J.S. reports personal fees from NovoNordisk, Jansen, Mundipharma, AstraZeneca. Esteve, Fresenius, Ingelheim Lilly, Vifor, ICU, Pfizer, Bayer, Travere Therapeutics, GE Healthcare and grants and personal fees from Boehringer Ingelheim, outside the current study. She is also member of the CKJ Editorial Board. C.G.-C. reports scientific advisory boards participation, grants and personal fees from Astra Zeneca, Esteve, Novonortis, Boehringer Ingelheim, Astellas, Otsuka, Novartis, Mundifarma, Baxter, Alexion, and Vifor. She is also member of the CKJ Editorial Board. G.I. reports consultancy and/or Honoraria from Novartis, Kite/Gilead, Bristol-Myers Squibb, Abbvie, Autolus, Sandoz, Miltenyi, AstraZeneca. P.B. reports consultancy: Allogene, Amgen, BMS, Gilead, Miltenyi biomedicine, Incyte, Novartis, Pfizer. DSMB (Clinical Trial): Miltenyi Biotec. Speaker: Amgen, BMS, Gilead, Novartis, Pfizer. Travel and accommodation: Gilead, Novartis, Pfizer.
REFERENCES
- 1. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018;379:64–73. 10.1056/nejmra1706169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Z, Wu Z, Liu Y et al. New development in CAR-T cell therapy. J Hematol Oncol 2017;10:53. 10.1186/s13045-017-0423-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eshhar Z. The T-body approach: redirecting T cells with antibody specificity. Handb Exp Pharmacol 2008;181:329–42. 10.1007/978-3-540-73259-4_14 [DOI] [PubMed] [Google Scholar]
- 4. Dai H, Wang Y, Lu X et al. Chimeric antigen receptors modified T-cells for ancer therapy. J Natl Cancer Inst 2016;108:djv439. 10.1093/jnci/djv439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med 2012;14:405–15. 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Locke FL, Miklos DB, Jacobson CA et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 2022;386:640–54. 10.1056/nejmoa2116133 [DOI] [PubMed] [Google Scholar]
- 7. Jhaveri KD, Rosner MH. Chimeric antigen receptor T cell therapy and the kidney. Clin J Am Soc Nephrol 2018;13:796–8. 10.2215/CJN.12871117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JH, Rivière I, Gonen M et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018;378:449–59. 10.1056/nejmoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davila ML, Riviere I, Wang X et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Turtle CJ, Hanafi LA, Berger C et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–38. 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maude SL, Laetsch TW, Buechner J et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/nejmoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. 10.1056/nejmoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fry TJ, Shah NN, Orentas RJ et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–8. 10.1038/nm.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee DW, Kochenderfer JN, Stetler-Stevenson M et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–28. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Porter DL, Hwang WT, Frey NV et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turtle CJ, Hay KA, Hanafi LA et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 2017;35:3010–20. 10.1200/JCO.2017.72.8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kochenderfer JN, Dudley ME, Kassim SH et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–9. 10.1200/JCO.2014.56.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuster SJ, Svoboda J, Chong EA et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54. 10.1056/nejmoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ali SA, Shi V, Maric I et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016;128:1688–700. 10.1182/blood-2016-04-711903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timmers M, Roex G, Wang Y et al. Chimeric antigen receptor-modified T cell therapy in multiple myeloma: beyond B cell maturation antigen. Front Immunol 2019;10:1613. 10.3389/fimmu.2019.01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma S, Li X, Wang X et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci 2019;15:2548–60. 10.7150/ijbs.34213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mougiakakos D, Krönke G, Völkl S et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med 2021;385:567–69. 10.1056/nejmc2107725 [DOI] [PubMed] [Google Scholar]
- 23. Brudno JN, Kochenderfer JN Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chakraborty R, Hill BT, Majeed A et al. Late effects after chimeric antigen receptor T cell therapy for lymphoid malignancies. Transplant Cell Ther 2020;27:222–9. 10.1016/j.jtct.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gust J, Hay KA, Hanafi LA et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 2017;7:1404–19. 10.1158/2159-8290.cd-17-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grupp SA, Kalos M, Barrett D et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–18. 10.1056/nejmoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riches JC, Davies JK, McClanahan F et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013;121:1612–21. 10.1182/blood-2012-09-457531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterner RM, Sakemura R, Cox MJ et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 2019;133:697–709. 10.1182/blood-2018-10-881722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casucci M, Hawkins RE, Dotti G et al. Overcoming the toxicity hurdles of genetically targeted T cells. Cancer Immunol Immunother 2015;64:123–30. 10.1007/s00262-014-1641-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuster SJ, Maziarz RT, Rusch ES et al. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Advances 2020;4:1432–9. 10.1182/bloodadvances.2019001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frey N, Porter D Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant 2019;25:e123–7. 10.1016/j.bbmt.2018.12.756. PMID: 30586620 [DOI] [PubMed] [Google Scholar]
- 32. Jhaveri KD, Rosner MH. Chimeric antigen receptor T cell therapy and the kidney what the nephrologist needs to know. Rosner Clin J Am Soc Nephrol 2018;13:796–8. 10.2215/CJN.12871117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gutgarts V, Jain T, Zheng J et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood Marrow Transplant 2020;26:1071–76. 10.1016/j.bbmt.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta S, Seethapathy H, Strohbehn IA et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis 2020;76:63–71. 10.1053/j.ajkd.2019.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kellum JA, Lameire N, Aspelin P et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2. 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 36. Lee DW, Santomasso BD, Locke FL et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahmoudjafari Z, Hawks KG, Hsieh AA et al. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group Survey on Chimeric Antigen Receptor T Cell Therapy Administrative, Logistic, and Toxicity Management Practices in the United States. Biol Blood Marrow Transplant 2019;25:26–33. 10.1016/j.bbmt.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 38. Hanna P, Strohbehn I, Moreno D et al. Comparison of short- and long-term adverse kidney outcomes after chimeric antigen receptor T cell therapy and autologous hematopoietic stem cell transplant for diffuse large B cell lymphoma. Bone Marrow Transplant 2022;57:1623–25; 10.1038/s41409-022-01767-7 [DOI] [PubMed] [Google Scholar]
- 39. Ahmed G, Bhasin B, Szabo A et al. Impact of chronic kidney disease and acute kidney injury on safety and outcomes of CAR T-cell therapy in lymphoma patients. Clin Lymphoma Myeloma Leuk 2022;22:863–8. 10.1016/j.clml.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 40. Farooqui N, Sy-Go JPT, Miao J et al. Incidence and risk factors for acute kidney injury after chimeric antigen receptor T-cell therapy. Mayo Clin Proc 2022;97:1294–304. 10.1016/j.mayocp.2022.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levey A, Eckardt K, Tsukamoto Y et al. Definition and classification of chronic kidney disease: A position statement from the Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–100. [DOI] [PubMed] [Google Scholar]
- 42. McEvoy GK, editor. American Hospital Formulary Service—Drug Information. Bethesda: American Society of Health-System Pharmacists; 2001, 972–8. [Google Scholar]
- 43. Lichtman SM, Etcubanas E, Budman DR et al. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest 2002;20:904–13. 10.1081/cnv-120005903 [DOI] [PubMed] [Google Scholar]
- 44. Bodge MN, Reddy S, Thompson MS et al. Preparative regimen dosing for hematopoietic stem cell transplantation in patients with chronic kidney disease: analysis of the literature and recommendations. Biol Blood Marrow Transplant 2014;20:908–19. 10.1016/j.bbmt.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 45. Golightly L, Teitelbaum I, Kizer TH et al. (eds) Renal pharma- cotherapy: dosage adjustment of medications eliminated by the kidneys. New York: Springer, 2013. [Google Scholar]
- 46. Aronoff GM, Bennett WM, Berns JS et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children. Am J Kidney Dis 1983;3:155–93. 10.1016/s0272-6386(83)80060-2 [DOI] [PubMed] [Google Scholar]
- 47. Wood AC, Perez AP, Arciola B et al. Outcomes of CD19-targeted chimeric antigen receptor T cell therapy for patients with reduced renal function including dialysis. Transplant Cell Ther 2022;28:829. 10.1016/j.jtct.2022.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khan I, Khan N, Wolfson N et al. Safety of CAR-T cell therapy in patients with renal failure/acute kidney injury: focused review. Clin Hematol Int 2023;5:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwon M, Iacoboni G, Reguera JL et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica 2023;108:110–21. 10.3324/haematol.2022.280805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lyu Y, Zhang M, Wei G et al. Risk factors of acute kidney injury during BCMA CAR-T cell therapy in patients with relapsed/refractory multiple myeloma. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022;51:137–43. 10.3724/zdxbyxb-2022-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. US Departament of Health and Human Services . Common terminology criteria for adverse events (CTCAE). 27 November 2017. Version 5.0.
- 52. Kanduri SR, Cheungpasitporn W, Thongprayoon C et al. Systematic review of risk factors and incidence of acute kidney injury among patients treated with CAR-T cell therapies. Kidney Int Rep 2021;6:1416–22. 10.1016/j.ekir.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chawla LS, Bellomo R, Bihorac A et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13:241–57. 10.1038/nrneph.2017.2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article itself.