Figure 1.
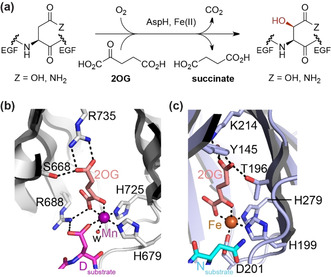
AspH catalyses a typical 2OG oxygenase reaction but has an atypical FeII binding mode, employing two rather than the typical three protein residues. a) The AspH reaction; b) view of the AspH FeII binding site (H679 and H725 complex the active site metal ion with Mn substituting for Fe; PDB: 6YYW) [8] compared with that of c) a human 2OG oxygenase with an FeII binding triad (i.e. H199, H279, and D201; PDB: 1H2L), [9] that is, the asparagine residue hydroxylase, factor inhibiting hypoxia‐inducible transcription factor HIF‐α (FIH).
