Figure 2.
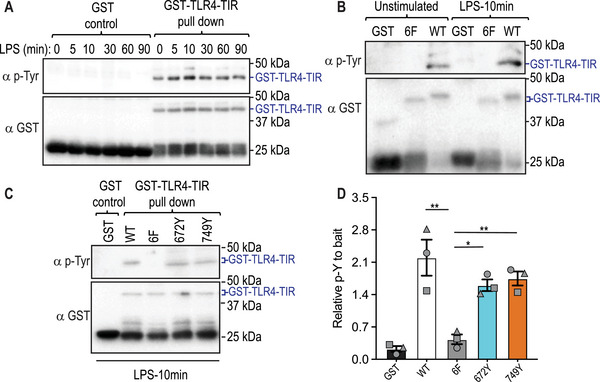
TLR4‐Y672 and Y749 are phosphorylated by extracts from LPS‐treated cells. (A‐D) A recombinant mouse TLR4‐TIR domain‐GST fusion protein was expressed in E. coli. GST‐TLR4‐TIR domains, alongside a GST only control, were purified prior to being incubated with lysates from RAW 264.7 cells that were stimulated with LPS (100 ng/mL) for the indicated time points. GST‐TLR4‐TIR domains were purified from the lysate mix and assessed for tyrosine phosphorylation via western blot. (A) Time‐dependent phosphorylation of the WT TLR4‐TIR domain. (B) WT GST‐TLR4‐TIR domain (WT) was compared alongside a mutant GST‐TLR4‐TIR protein in which six tyrosine residues (Y672F, Y678F, Y707F, Y749F, Y784F, Y792F) were mutated to phenylalanine (6F). (C) Tyrosine 672 (Y672) and 749 (Y749) were reintroduced into the 6F mutant and assessed, alongside WT and 6F TIR domains, for tyrosine phosphorylation upon incubation with lysates of LPS‐activated RAW 264.7 cells. (D) Quantification of western blots from panel (C) for total tyrosine phosphorylation, relative to total levels of relevant GST protein. Data are combined from three independent experiments (mean ± SEM, n = 3), with each symbol representing a different experiment. Statistical analyses were performed using a repeated measures one‐way ANOVA, followed by Bonferroni's multiple comparison test (*p < 0.05, **p < 0.01).
