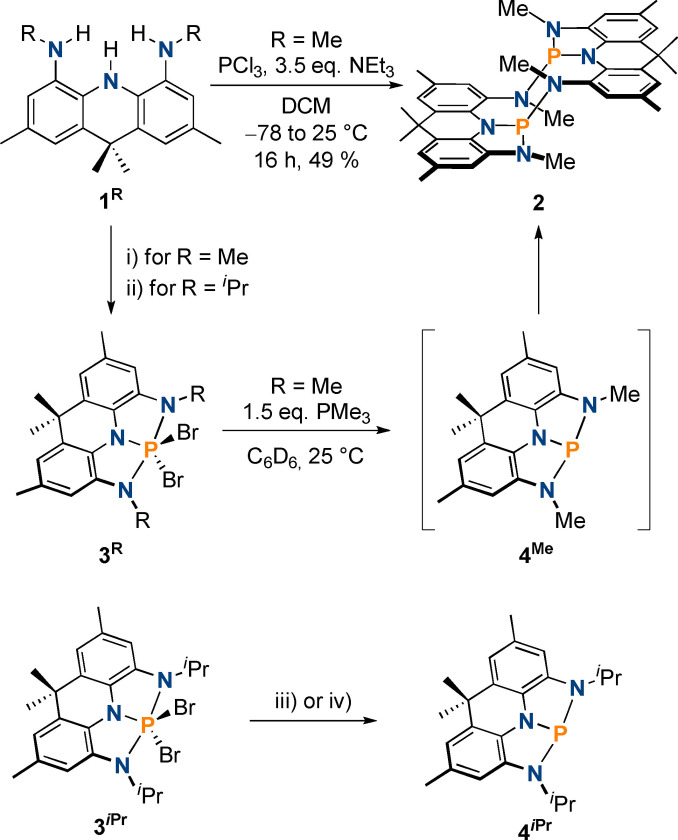
Scheme 1.
Reactivity of the protio‐ligand 1R towards PCl3 and PBr5 in the presence of NEt3 to generate 2 and 3R as well as conversion of 3Me towards 2 via transient 4Me (top); Generation of 4 i Pr from 3 i Pr upon dehalogenation with PMe3 or Mg (bottom); i) PBr5, 3 equiv. NEt3, toluene, −78 to 25 °C, 16 h, 64 % isolated yield; ii) PBr5, 5 equiv. NEt3, toluene, −78 to 25 °C, 16 h, 54 % isolated yield; iii) 5 equiv. PMe3, C6H6, 25 °C, 15 min, 90 % isolated yield; iv) 20 equiv. Mg, THF, 25 °C, 30 min, 90 % isolated yield.