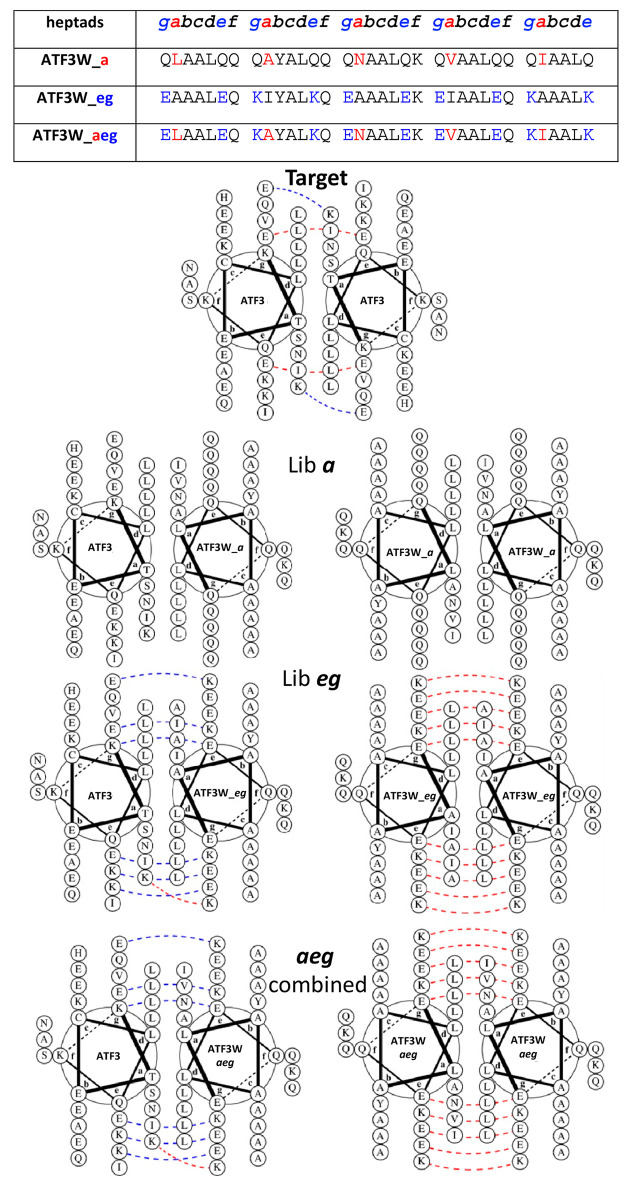
Figure 5.
Helical wheel representations of potential interactions with the PCA selected ATF3W_a and ATF3W_eg sequences as well as combined sequence ATF3W_aeg. ATF3-ATF3W_aeg heterodimeric and ATF3W_aeg homodimeric helical wheel diagrams show hydrophobic residues at core positions (a/d) as well as charged residues present at the surrounding positions (e/g). The d positions were held as Leu in order to maintain the leucine zipper structure. The ATF3-ATF3W_aeg interaction contains favorable electrostatic (blue dashed line) and core interactions to drive formation of the coiled coil. In contrast, the ATF3W_aeg dimer displays unfavorable electrostatic interactions (red dashed line) and van der Waals interactions with the core, disfavoring its formation. Helical wheel diagrams were generated using DrawCoil 1.0, https://grigoryanlab.org/drawcoil/.11