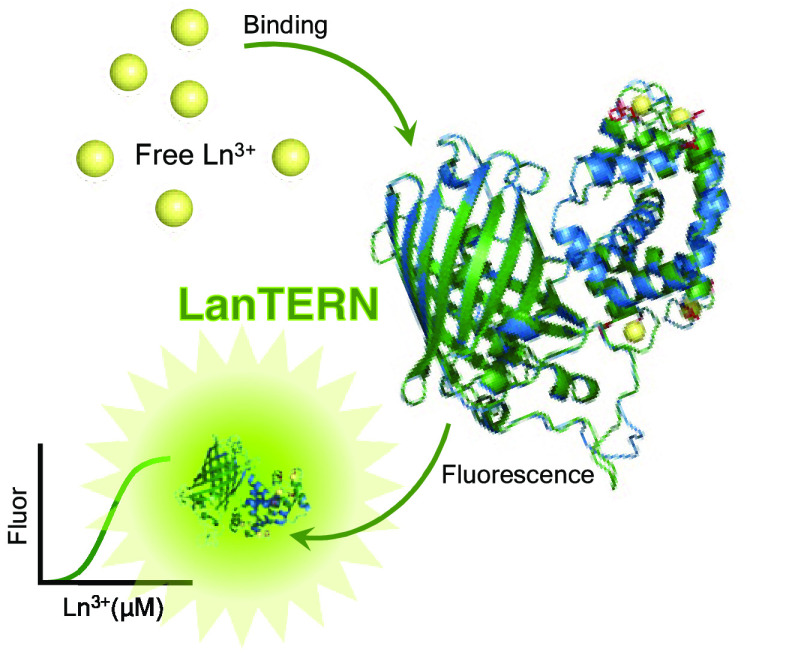
Abstract
Lanthanides, a series of 15 f-block elements, are crucial in modern technology, and their purification by conventional chemical means comes at a significant environmental cost. Synthetic biology offers promising solutions. However, progress in developing synthetic biology approaches is bottlenecked because it is challenging to measure lanthanide binding with current biochemical tools. Here we introduce LanTERN, a lanthanide-responsive fluorescent protein. LanTERN was designed based on GCaMP, a genetically encoded calcium indicator that couples the ion binding of four EF hand motifs to increased GFP fluorescence. We engineered eight mutations across the parent construct’s four EF hand motifs to switch specificity from calcium to lanthanides. The resulting protein, LanTERN, directly converts the binding of 10 measured lanthanides to 14-fold or greater increased fluorescence. LanTERN development opens new avenues for creating improved lanthanide-binding proteins and biosensing systems.
Keywords: rare-earth element, lanthanide, lanmodulin, EF hand, LanM, GCaMP
Introduction
Modern technology relies on lanthanides, a series of 15 f-block elements. Lanthanides have similar physicochemical properties and co-occur within the Earth’s crust but must be separated for use in technological applications. Separation of lanthanides is environmentally costly and typically requires tens or hundreds of stages of solvent extraction.1 Given these elements’ critical importance, new, cost-effective, environmentally friendly methods of lanthanide separation are needed.
Natural microbes contain protein-based machinery that binds individual lanthanides with varying affinities. For example, some methanotrophic organisms preferentially import light lanthanides and use them as cofactors for alcohol dehydrogenases.2 Many of these organisms also contain lanmodulins (LanMs). LanMs are a family of proteins that use distinct variants of the EF hand motif to specifically bind to lanthanides, with a slight preference for the lighter lanthanides.2−4 Recently, LanMs were used to help separate mixtures of lanthanides such as neodymium and dysprosium.3,4 Synthetic biology offers the potential to develop enhanced molecules and organisms that bind to, discriminate between, and thereby separate different lanthanide elements.
Synthetic biology can engineer proteins with novel or improved functionality, such as binding, provided that those functions can be measured. However, our ability to measure the binding of lanthanides to proteins is limited. Currently, most lanthanide binding measurements rely on sensitized terbium emission, which suffers several limitations: it only directly detects a single lanthanide element, requires luminescence which is incompatible with flow cytometers and many plate readers, and imposes the design requirement of a proximal tryptophan.5,6 While one FRET-based sensor detects lanthanides by conformational change of the native LanM protein, no published fluorophore sensor directly transduces the binding of any lanthanide ion other than terbium into a fluorescent output.7 Here we describe a lanthanide sensor, LanTERN, that directly couples generic lanthanide binding to a change in the green fluorescent protein (GFP) signal.
Results
We designed our fluorescent sensor based on GCaMP, a genetically encoded calcium indicator. GCaMP has been extensively optimized as a reporter of intracellular calcium concentration in the context of live-cell imaging.8 Given the chemical similarity between the Ca2+ and Ln3+ ions, we planned to create a fluorescent sensor that could be used to measure the binding of lanthanides in vitro by switching the specificity of the GCaMP from calcium to lanthanides.
GCaMP is a fusion protein that comprises an N-terminal calmodulin-binding peptide (CBP), a circularly permuted green fluorescent protein (cpGFP), and a C-terminal calmodulin. Calmodulin, in turn, is composed of four 12 amino acid calcium-binding motifs (EF hands) separated by 24- or 25-amino acid linkers.9 In the presence of calcium, calmodulin undergoes a marked conformational change, exposing an internal binding site for CBP to bind in cis. This intramolecular binding increases the brightness of GFP by excluding water from its chromophore.10
The conformational shift by calmodulin is driven by the binding of calcium ions to its four EF hand motifs. To switch GCaMP’s specificity from calcium to lanthanides, we used the EF hands of Methylorubrum extorquens LanM (Mex-LanM) to construct a lanthanide-responsive superfolder GCaMP variant by combining an M13 CBP and a circularly permuted superfolder GFP with a chimeric Rattus norvegicus calmodulin in which each of the four EF hands was replaced with the corresponding EF hand from Mex-LanM (Figure 1a, middle, and Figure 1b, blue).
Figure 1.

Rational engineering of EF hand motifs converts GCaMP into a lanthanide sensor. (A) Sequences of EF hands 1, 2, 3, and 4 of GCaMP, LanM-GCaMP, and LanTERN. Amino acids identical to GCaMP are green; amino acids derived from Mex-LanM and not found in GCaMP are blue; intervening linkers (not to scale) are depicted as lines. Red stars indicate amino acid side chains shown as red sticks in (B). (B) Overlaid models of metal-bound LanM-GCaMP (blue) and LanTERN (green). Prolines at EF hand position 2 and putative lanthanide-binding aspartates at EF hand position 9 are shown as red sticks. (C) Fluorescence measurements of 500 nM LanM-GCaMP (see Figure 1A, middle and Figure 1B, blue) in the presence of varying calcium, lanthanum, and ytterbium concentrations. Points and error bars represent the mean and standard deviation of three technical replicates from the same protein purification and working dilution. Graphs of two additional protein purifications can be found in Supplemental Figure 3. (D) Fluorescence measurements of 500 nM LanTERN (see Figure 1A, bottom, and Figure 1B, green) in varying lanthanum, ytterbium, and calcium concentrations. Points and error bars represent the mean and standard deviation of three technical replicates from the same protein purification and working dilution. Graphs of two additional protein purifications can be found in Supplemental Figure 4.
We expressed this construct, termed LanM-GCaMP, in Escherichia coli, purified it using Ni-NTA chromatography (see Supplemental Methods), and measured its dose–response in vitro to lanthanum, the lightest lanthanide, ytterbium, the heaviest non-d-block lanthanide, and calcium. Installation of the EF hands from LanM increased brightness of the GFP in the presence of lanthanides (approximately 10 μM to 1 mM) but not in the presence of calcium (Figure 1c). This stands in contrast to the Kd of Mex-LanM, which is reported to be in the picomolar range.3 We reasoned that the relatively weak response might have been due to the orientation of the hands in the calmodulin backbone; EF hands are paired in the ion-bound conformation, and LanM and calmodulin differ in their arrangement.3,9 We tested three alternative hand arrangements to mimic the native arrangement in Mex-LanM but did not observe improved performance (Supplemental Figure 1).
We reasoned that we could improve the sensor’s performance by installing the features of the LanM EF hands that are thought to confer lanthanide selectivity3,4 in the native calmodulin EF hands. In Mex-LanM, the proline residue at the second position of each EF hand is critical to specificity. Mutating these proline residues ablates Mex-LanM’s specificity for lanthanides versus calcium;3 they are conserved across LanMs.4 Therefore, we mutated the second position of each EF hand to a proline. We also mutated each of the first four metal-contacting residues (EF hand residues 1, 3, 5, and 9) to aspartic acid because in Mex-LanM these residues either directly coordinate lanthanides (residues 1, 3, and 5) or hydrogen-bond to a lanthanide-coordinating water molecule (residue 9).3,4,11 The resulting construct was termed LanTERN, for lanthanide-tuned EF hand reporter fluorescent (Figure 1a, bottom; Figure 1b, green).
We measured the dose–response of LanTERN to lanthanides. The concentration of lanthanides that yielded the maximal response (Lnmax) varied among lanthanides (0.5 μM–10 μM). Above Lnmax, we observed nonmonotonic behavior in the fluorescence (Supplemental Figure 6). Therefore, we defined LanTERN’s dynamic range as [Ln] < Lnmax and restricted our characterization and analysis to this range.
Within LanTERN’s dynamic range, we calculated EC50 values for LanTERN to be 976 nM for lanthanum and 4.71 μM for ytterbium, with no measurable response to calcium (Supplemental Table 1), copper(II), cobalt(II), iron(II), magnesium, or manganese (Supplemental Figure 9). This is an improvement of greater than 2 orders of magnitude over LanM-GCaMP. LanTERN’s lanthanide-dependent increase in fluorescence versus baseline also increased over LanM-GCaMP’s (e.g., >10-fold change vs ∼3.5-fold change in response to lanthanum) (Figure 1d). We also measured the apparent Kd of LanTERN for La3+ using chelator-buffered titrations12 and calculated it to be 40–60 pM, which is in line with other work on proteins containing LanM EF hands (Supplemental Figure 11).3,7
Importantly, our LanTERN design also switches the function of GCaMP. We found that LanTERN does not respond to calcium, as its response to lanthanides was nearly identical in the presence of calcium concentrations as high as 500 μM (Supplemental Figure 10). To confirm the importance of the proline residue in the second position of each engineered EF hand, we created a LanTERN variant where the second position proline was back-mutated to the cognate amino acid found in wild-type calmodulin. As expected, these mutations reduced the sensor’s response to lanthanides by approximately 10-fold and restored its response to calcium (Supplemental Figure 2).
Finally, we characterized LanTERN’s response to 10 lanthanides that span the atomic weight of this class. LanTERN responds to all lanthanides tested: we observed a 14-fold or greater lanthanide-dependent fluorescence increase versus baseline (Figure 2). The sensor exhibited binding preferences similar to Mex-LanM, generally responding at lower concentrations of the lighter lanthanides (Figure 2B).3,4 The difference in EC50 values for the lightest and heaviest lanthanides tested differed by approximately 4-fold. However, LanTERN’s EC50 values are on the order of the concentration of the sensor, meaning that LanTERN’s actual affinity for the lanthanides is likely much higher.
Figure 2.

LanTERN responds to all tested lanthanides. (A) Fluorescence measurements of 500 nM LanTERN in the presence of varying concentrations of lanthanides listed in order of atomic mass. Points and error bars represent the mean and standard deviation of three technical replicates from the same protein purification and working dilution. Lines represent a linear interpolation between points. Graphs of two additional protein purifications can be found in Supplemental Figure 8. (B) Table of calculated EC50values of LanTERN in response to lanthanides. Lanthanides are shown in order of atomic number (Z). Values represent the mean ± standard deviations of three independent protein purifications of LanTERN. Values for the individual protein purifications can be found in Supplemental Table 1.
Discussion
In this study, we report the construction of LanTERN, a lanthanide-responsive fluorescent protein. We rationally engineered EF hand motifs to build a fluorescent protein sensor with switched specificity for lanthanides versus calcium. This capability opens new avenues for the creation of improved lanthanide-binding proteins. LanTERN could be used as a sensing tool in directed evolution studies to identify mutations in EF hands that increase the selectivity and affinity for specific lanthanides. For example, improved variants of LanTERN could be found using yeast surface display and fluorescence-activated cell sorting, which is not possible using luminescence sensors based on terbium. These improved binders could be used in synthetic biological approaches for separating lanthanides.
Alternatively, LanTERN could also be used to develop engineered calmodulin domains for biosensing systems. LanTERN’s M13 and engineered calmodulin domains could be split and used as lanthanide-dependent heterodimeric binders. Each of these domains could then be attached to components of a dimerization-based cell control system. Such systems could then utilize the lanthanide-dependent dimerization of M13 and LanTERN calmodulin to create a lanthanide-dependent response for cellular functions such as transcription13,14 or phosphorylation15 or to create luminescence-based sensors.16 These systems, especially when combined with calmodulins from improved and more specific LanTERN variants, would enable the creation of organisms that respond to the presence of lanthanides and assist in the extraction and separation of lanthanides.
Methods
Detailed protocols for all methods used in this report are given in the Supporting Information. Annotated sequences for all constructs used are listed in the SI zip file. A T7 bacterial expression construct for LanTERN is available on Addgene (Addgene ID 214061).
Acknowledgments
pRSET sfGCaMP6s-T78H was a gift from Wolf Frommer (Addgene plasmid #100023). The authors thank the Laboratory of Systems Pharmacology at Harvard Medical School for access to their equipment and Neil Dalvie for his helpful comments on the manuscript. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant DGE 2140743. This work was supported by funds from the MITRE Corporation, the Wyss Institute for Biologically Inspired Engineering, and the Synthetic Biology HIVE at Harvard Medical School.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssynbio.3c00600.
Supplemental methods and protocols for experiments and analysis performed in this report; La, Yb, and Ca dose–response of LanM-GCaMP variants with different LanM hand ordering (Supplemental Figure 1); La, Yb, and Ca dose–response of LanTERN with back mutations in the second position of each EF hand (Supplemental Figure 2); La, Yb, and Ca dose–response of LanM-GCaMP, including all three independent protein purifications (Supplemental Figure 3); La, Yb, and Ca dose–response of LanTERN, including all three independent protein purifications (Supplemental Figure 4); La, Yb, and Ca dose–response of LanM-GCaMP variants with different LanM hand orderings, including all three independent protein purifications (Supplemental Figure 5); La, Yb, and Ca dose–response of LanTERN showing nonmonotonic dynamics outside of Lnmax (Supplemental Figure 6); La, Yb, and Ca dose–response of LanTERN with back mutations in the second position of each EF hand, including all three independent protein purifications (Supplemental Figure 7); dose–response of LanM-GCaMP to 10 lanthanides, including all three independent protein purifications (Supplemental Figure 8); LanTERN response to nonlanthanide metals (Supplemental Figure 9); LanTERN is not inhibited by calcium (Supplemental Figure 10); lanthanum chelator-buffered titration using EDDS (Supplemental Figure 11); calculated EC50 values for LanTERN from Figure 1D (Supplemental Table 1); oligonucleotides used in this study (Supplemental Table 2); constructs used in this study (Supplemental Table 3); list of catalog numbers for materials used in this report (PDF)
Raw and processed data from each experiment in this report; jupyter notebooks used to analyze and plot these data; annotated GenBank files for constructs used in this report; .pdb files of structures shown in Figure 2 (ZIP)
Author Contributions
E.M.J., P.A.S., Q.A.J., and M.S. conceptualized project direction. E.M.J. conceptualized, designed, and cloned sensors, planned and conducted experiments, and analyzed data. Y.S. constructed the protein structure models of LanM-GCaMP and LanTERN. E.M.J., Q.A.J., and Y.S. created figures. E.M.J. wrote the manuscript. E.M.J., P.A.S., and Q.A.J. edited the manuscript. All authors approved the manuscript.
The authors declare the following competing financial interest(s): C.S. is on the scientific advisory board of Cytoreason.com.
Supplementary Material
References
- Cheisson T.; Schelter E. J. Rare Earth Elements: Mendeleev’s Bane, Modern Marvels. Science 2019, 363 (6426), 489–493. 10.1126/science.aau7628. [DOI] [PubMed] [Google Scholar]
- Pol A.; Barends T. R. M.; Dietl A.; Khadem A. F.; Eygensteyn J.; Jetten M. S. M.; Op den Camp H. J. M. Rare Earth Metals Are Essential for Methanotrophic Life in Volcanic Mudpots. Environ. Microbiol. 2014, 16 (1), 255–264. 10.1111/1462-2920.12249. [DOI] [PubMed] [Google Scholar]
- Cotruvo J. A.; Featherston E. R.; Mattocks J. A.; Ho J. V.; Laremore T. N. Lanmodulin: A Highly Selective Lanthanide-Binding Protein from a Lanthanide-Utilizing Bacterium. J. Am. Chem. Soc. 2018, 140 (44), 15056–15061. 10.1021/jacs.8b09842. [DOI] [PubMed] [Google Scholar]
- Mattocks J. A.; Jung J. J.; Lin C.-Y.; Dong Z.; Yennawar N. H.; Featherston E. R.; Kang-Yun C. S.; Hamilton T. A.; Park D. M.; Boal A. K.; Cotruvo J. A. Enhanced Rare-Earth Separation with a Metal-Sensitive Lanmodulin Dimer. Nature 2023, 618 (7963), 87–93. 10.1038/s41586-023-05945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz M.; Franz K. J.; Maglathlin R. L.; Imperiali B. A Powerful Combinatorial Screen to Identify High-Affinity Terbium(III)-Binding Peptides. ChemBioChem 2003, 4 (4), 272–276. 10.1002/cbic.200390047. [DOI] [PubMed] [Google Scholar]
- Featherston E. R.; Issertell E. J.; Cotruvo J. A. Jr Probing Lanmodulin’s Lanthanide Recognition via Sensitized Luminescence Yields a Platform for Quantification of Terbium in Acid Mine Drainage. J. Am. Chem. Soc. 2021, 143 (35), 14287–14299. 10.1021/jacs.1c06360. [DOI] [PubMed] [Google Scholar]
- Mattocks J. A.; Ho J. V.; Cotruvo J. A. A Selective, Protein-Based Fluorescent Sensor with Picomolar Affinity for Rare Earth Elements. J. Am. Chem. Soc. 2019, 141 (7), 2857–2861. 10.1021/jacs.8b12155. [DOI] [PubMed] [Google Scholar]
- Akerboom J.; Rivera J. D. V.; Guilbe M. M. R.; Malavé E. C. A.; Hernandez H. H.; Tian L.; Hires S. A.; Marvin J. S.; Looger L. L.; Schreiter E. R. Crystal Structures of the GCaMP Calcium Sensor Reveal the Mechanism of Fluorescence Signal Change and Aid Rational Design. J. Biol. Chem. 2009, 284 (10), 6455–6464. 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford J. L.; Walsh M. P.; Vogel H. J. Structures and Metal-Ion-Binding Properties of the Ca2+-Binding Helix-Loop-Helix EF-Hand Motifs. Biochem. J. 2007, 405 (2), 199–221. 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Shui B.; Kotlikoff M. I.; Sondermann H. Structural Basis for Calcium Sensing by GCaMP2. Struct. London Engl. 1993 2008, 16 (12), 1817–1827. 10.1016/j.str.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E. C.; Featherston E. R.; Showalter S. A.; Cotruvo J. A. Structural Basis for Rare Earth Element Recognition by Methylobacterium Extorquens Lanmodulin. Biochemistry 2019, 58 (2), 120–125. 10.1021/acs.biochem.8b01019. [DOI] [PubMed] [Google Scholar]
- Mattocks J. A.; Tirsch J. L.; Cotruvo J. A. Chapter Two - Determination of Affinities of Lanthanide-Binding Proteins Using Chelator-Buffered Titrations. Methods Enzymol. 2021, 651, 23–61. 10.1016/bs.mie.2021.01.044. [DOI] [PubMed] [Google Scholar]
- Pu J.; Zinkus-Boltz J.; Dickinson B. C. Evolution of a Split RNA Polymerase as a Versatile Biosensor Platform. Nat. Chem. Biol. 2017, 13 (4), 432–438. 10.1038/nchembio.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-J.; Mayonove P.; Zavala A.; De Visch A.; Minard P.; Cohen-Gonsaud M.; Bonnet J. A Modular Receptor Platform To Expand the Sensing Repertoire of Bacteria. ACS Synth. Biol. 2018, 7 (1), 166–175. 10.1021/acssynbio.7b00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazé A.; Benenson Y. Artificial Signaling in Mammalian Cells Enabled by Prokaryotic Two-Component System. Nat. Chem. Biol. 2020, 16 (2), 179–187. 10.1038/s41589-019-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. P.; Unch J.; Binkowski B. F.; Valley M. P.; Butler B. L.; Wood M. G.; Otto P.; Zimmerman K.; Vidugiris G.; Machleidt T.; Robers M. B.; Benink H. A.; Eggers C. T.; Slater M. R.; Meisenheimer P. L.; Klaubert D. H.; Fan F.; Encell L. P.; Wood K. V. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7 (11), 1848–1857. 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.