Figure 2. CR3-dependent phagocytosis of Opa-negative N. gonorrhoeae by adherent neutrophils.
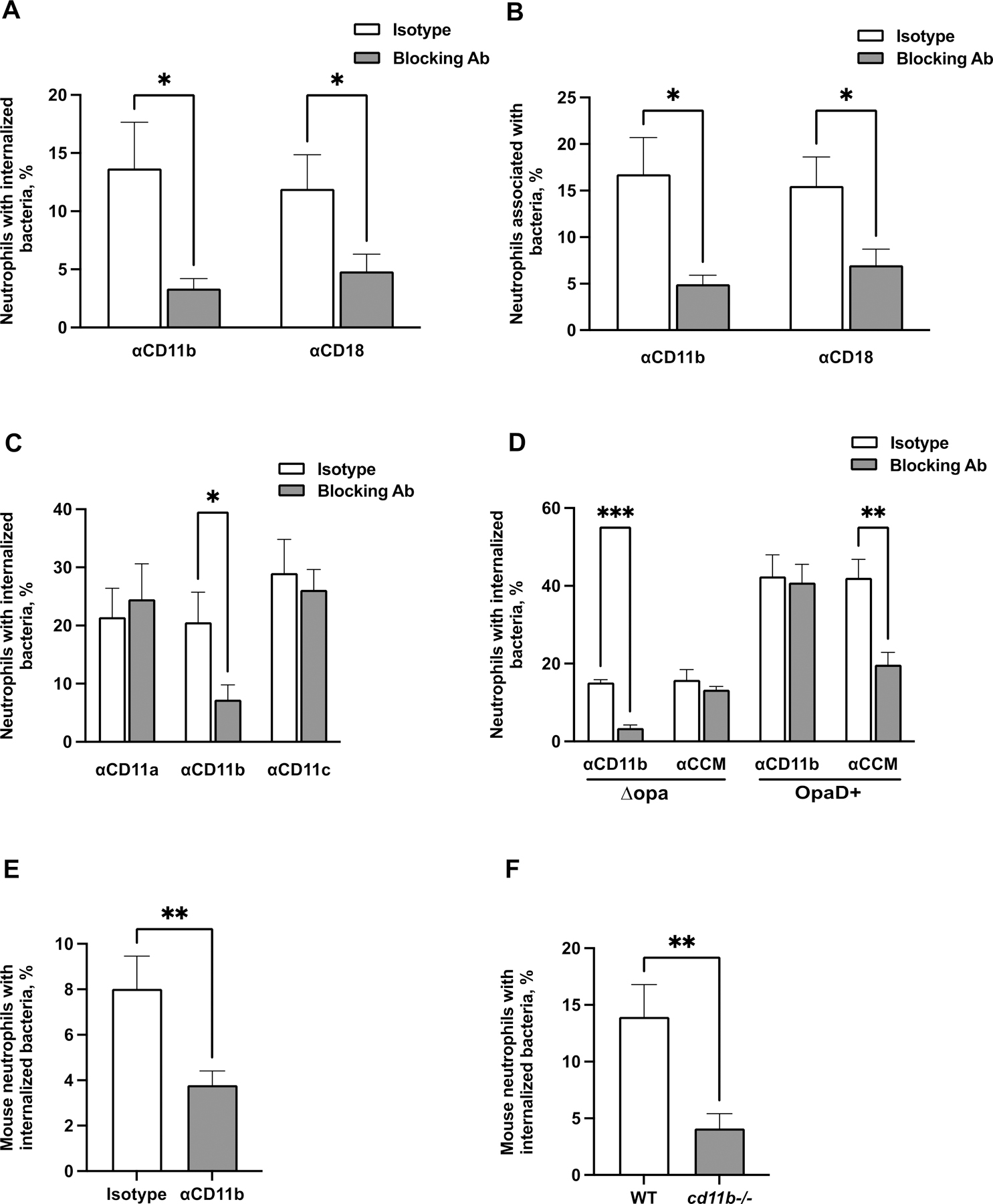
(A-B) Adherent, IL-8 treated primary human neutrophils were exposed to blocking antibodies against CD11b (44a) or CD18 (TS1/18) (gray bars), or matched isotype control (white bars). Neutrophils were infected with CFSE-labeled Δopa Ngo for 1 hr, fixed, and stained for extracellular Ngo using a DyLight™ 650 (DL650)-labeled antibody without permeabilization. A step gate was applied to quantify the percentage of neutrophils with internalized (A) and associated (bound and internalized) (B) Ngo. (C) Neutrophils were treated with blocking antibodies against CD11a, CD11b, or CD11c, or isotype control. Internalization of Δopa Ngo was measured as above. (D) Neutrophils were treated with blocking antibodies against human CEACAMs (CCM) or CD11b (44a). Internalization of Δopa or OpaD+ Ngo was measured as above. (E-F) Neutrophils were purified from the bone marrow of WT or cd11b-/- C57BL/6J mice. In E, adherent neutrophils from WT mice were exposed to anti-CD11b blocking antibody (M170) or isotype control. Neutrophils were incubated with Tag-IT Violet® labeled Δopa Ngo for 1 hr, fixed, and stained for extracellular bacteria with DL650-labeled anti-Ngo antibody and for the neutrophil surface with FITC-coupled antibody against Ly6G. Cells were analyzed by imaging flow cytometry as above (see Fig. S5A for mouse neutrophil gating strategy), and the percent of mouse neutrophils with intracellular bacteria was calculated. Results presented are the mean ± SEM of the following number of biological replicates: (A-B) 4–5; (C), 3–4; (D), 4; (E-F), 6. In A-F, statistical significance was determined by Student’s paired t test. *, P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001.
