Fig. 4. Human neutrophils are not a source of C3 for opsonization of N. gonorrhoeae.
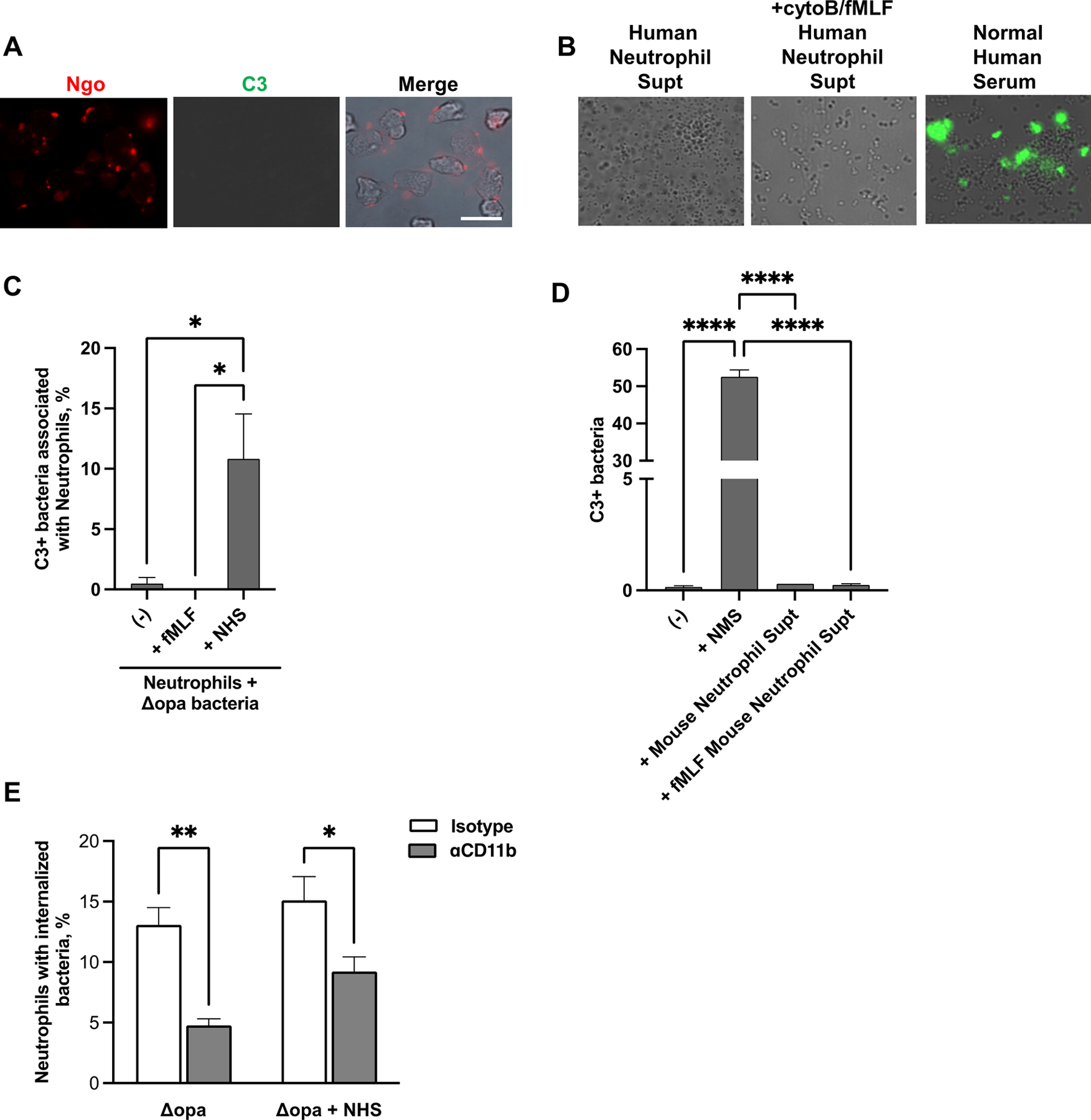
(A) Adherent, IL-8 treated primary human neutrophils were exposed to non-opsonized Δopa Ngo for 15 min, fixed, and stained with FITC-labeled anti-C3 (green) and DL650-labled anti-Ngo (red) antibodies. Fluorescence micrographs were captured for the FITC and DL650 channels (presented as grayscale), with the merge in color showing overlap with the phase image for the neutrophils. (B) Δopa Ngo was incubated with normal human serum as a positive control for C3 opsonization (third panel), or with the supernatants collected from adherent primary human neutrophils that were left untreated (first panel) or treated with cytochalasin B (cytoB) and fMLF to stimulate degranulation (second panel). Bacteria were washed and stained with FITC-labeled anti-C3 antibody. Representative fluorescence micrographs with the FITC channel overlaid on the phase image are presented. (C) Neutrophils were treated with cytoB + fMLF as above, or left untreated (-). Neutrophils were exposed for 15 min to unopsonized Δopa Ngo. Neutrophils were fixed, stained with FITC-labeled anti-C3 antibody and DL650-labeled anti-Ngo antibodies, and analyzed by imaging flow cytometry. The spot count feature was used to quantify cells with positive FITC signals within the population of neutrophils containing 1–3 DL650+ Ngo for 3 biological replicates. Untreated neutrophils exposed to Δopa Ngo that was opsonized in normal human serum (ops) served as a positive control. (D) Δopa Ngo was left untreated, or incubated with the supernatant from adherent mouse neutrophils that were untreated (Mouse Neutrophil Supt) or treated with cytoB + fMLF (+fMLF Mouse Neutrophil Supt). Ngo was incubated with 50% normal mouse serum as a positive control (+NMS) or PBS as a negative control (-). Ngo was washed and stained with FITC-labeled anti-mouse C3 antibody and analyzed by imaging flow cytometry as in C. The percent of bacteria that were in the C3+ gate was quantified from 3 biological replicates. (E) Adherent, IL-8 treated primary human neutrophils were exposed to anti-CD11b (44a; gray bars) or isotype control (white bars), then infected with Δopa Ngo that was not opsonized (left) or opsonized in normal human serum as in B (right). The percent of neutrophils with internalized Ngo was quantified by imaging flow cytometry as in Fig. 2. Results presented are for 4–6 biological replicates. Statistical significance in C-D was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test, and in E by paired Student’s t test. * P ≤ 0.05,** P ≤ 0.01***, P ≤ 0.001, **** P ≤ 0.0001.
