Figure 5. Active CR3 promotes phagocytosis of Opa-negative N. gonorrhoeae by human neutrophils in suspension.
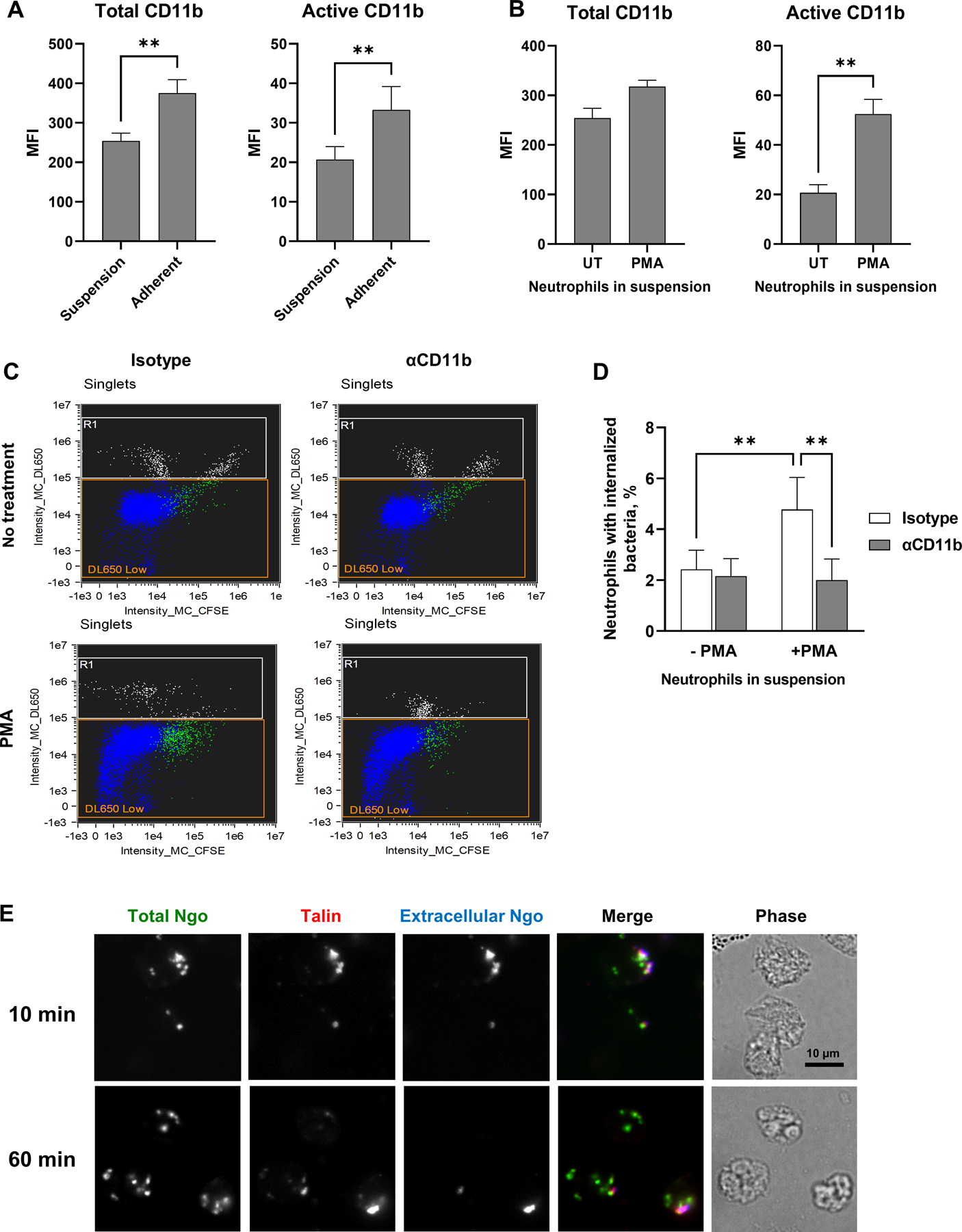
(A) Human neutrophils were maintained in suspension or allowed to adhere to coverslips in the presence of IL-8. The median fluorescence intensity (MFI) of total (left) and active (right) CD11b on the neutrophil surface from n = 5 biological replicates is presented. (B) Neutrophils in suspension were left untreated (UT) or treated with PMA, then stained for total (ICRF44; left) and active (CBRM1/5; right) CD11b. The MFI from n= 5 biological replicates is presented. (C-D) Untreated or PMA-treated neutrophils in suspension were exposed to anti-CD11b antibody or isotype control, then infected with CFSE-labeled Δopa Ngo at MOI = 1. Phagocytosis was measured by imaging flow cytometry as in Fig. 2. (C) Dot plots of the intensity of CFSE (total Ngo) vs. intensity of DL650 (extracellular Ngo). Intact, single neutrophils are identified as the DL650 Low population (blue; R1 = gate for DL650hi cells that are not intact). The green color identifies the population of neutrophils that are associated with CFSE+ Ngo. (D) The percentages of untreated and PMA-treated neutrophils with internalized bacteria were calculated. Results presented are the mean ± SEM from n=4 biological replicates. (E) Adherent, IL-8 treated human neutrophils were exposed to CFSE+ Δopa Ngo (green) for 10 min to allow for attachment, or 60 min to allow for internalization. Cells were fixed, stained for extracellular Ngo (blue), then permeabilized and stained for talin (red). Colocalization between extracellular, cell-associated Ngo and talin is seen in the 10 min merged image. Statistical significance was determined by paired Student’s t test (A-B) or two-way ANOVA followed by Sidak’s multiple comparison test (D) for 5 biological replicates. ** P ≤ 0.01.
