Abstract
BACKGROUND:
Antimicrobial envelopes reduce the incidence of cardiac implantable electronic device infections, but their cost restricts routine use in the United Kingdom. Risk scoring could help to identify which patients would most benefit from this technology.
METHODS:
A novel risk score (BLISTER [Blood results, Long procedure time, Immunosuppressed, Sixty years old (or younger), Type of procedure, Early re-intervention, Repeat procedure]) was derived from multivariate analysis of factors associated with cardiac implantable electronic device infection. Diagnostic utility was assessed against the existing PADIT score (Prior procedure, Age, Depressed renal function, Immunocompromised, Type of procedure) in both standard and high-risk external validation cohorts, and cost-utility models examined different BLISTER and PADIT score thresholds for TYRX (Medtronic; Minneapolis, MN) antimicrobial envelope allocation.
RESULTS:
In a derivation cohort (n=7383), cardiac implantable electronic device infection occurred in 59 individuals within 12 months of a procedure (event rate, 0.8%). In addition to the PADIT score constituents, lead extraction (hazard ratio, 3.3 [95% CI, 1.9–6.1]; P<0.0001), C-reactive protein >50 mg/L (hazard ratio, 3.0 [95% CI, 1.4–6.4]; P=0.005), reintervention within 2 years (hazard ratio, 10.1 [95% CI, 5.6–17.9]; P<0.0001), and top-quartile procedure duration (hazard ratio, 2.6 [95% CI, 1.6–4.1]; P=0.001) were independent predictors of infection. The BLISTER score demonstrated superior discriminative performance versus PADIT in the standard risk (n=2854, event rate: 0.8%, area under the curve, 0.82 versus 0.71; P=0.001) and high-risk validation cohorts (n=1961, event rate: 2.0%, area under the curve, 0.77 versus 0.69; P=0.001), and in all patients (n=12 198, event rate: 1%, area under the curve, 0.8 versus 0.75, P=0.002). In decision-analytic modeling, the optimum scenario assigned antimicrobial envelopes to patients with BLISTER scores ≥6 (10.8%), delivering a significant reduction in infections (relative risk reduction, 30%; P=0.036) within the National Institute for Health and Care Excellence cost-utility thresholds (incremental cost-effectiveness ratio, £18 446).
CONCLUSIONS:
The BLISTER score (https://qxmd.com/calculate/calculator_876/the-blister-score-for-cied-infection) was a valid predictor of cardiac implantable electronic device infection, and could facilitate cost-effective antimicrobial envelope allocation to high-risk patients.
Keywords: cardiac resynchronization therapy, defibrillators, heart failure, infections, quality of life, risk factors
WHAT IS KNOWN?
The incidence of cardiac implantable electronic device infection is rising.
The WRAP-IT trial demonstrated that the TYRX antimicrobial envelope reduces the incidence of cardiac implantable electronic device infection at 12 months post-intervention in selected high-risk patients.
Given the high cost of the envelope, there is a clear need for a discerning and quantitative method to govern cost-effective use
WHAT THE STUDY ADDS
The BLISTER score is a novel, externally validated predictor of infection in a real-world cardiac implantable electronic device population.
Cost-utility modeling suggests a BLISTER score threshold of ≥6 could be used to allocate antimicrobial envelopes within established willingness-to-pay thresholds.
Cardiac implantable electronic device (CIED) infection is a serious complication of device therapy, with significant ramifications for patient morbidity, mortality, quality of life, and health care costs.1,2 The incidence of CIED infection is rising, attributed to the increasing use of complex devices, successive reinterventions on device pockets, and the proliferation of predisposing comorbidities.3–5
The WRAP-IT randomized controlled trial (Worldwide Randomized Antibiotic Envelope Infection Prevention Trial) demonstrated how use of the TYRX antimicrobial envelope (AE) during cardiac resynchronization therapy-defibrillator (CRT-D) implant or device reintervention reduced the risk of infection at 12 months, and this technology has since been adopted into the European Heart Rhythm Association guidelines for high-risk patients.6,7 However, the current European Heart Rhythm Association definition of “high-risk” incorporates a large proportion of the CIED population (including those with dual chamber devices, heart failure, or diabetes) and, given the high cost of the AE, strict adherence with these recommendations may not conform with policymakers’ cost-utility thresholds. In the United Kingdom, the National Institute for Health and Care Excellence (NICE) technology appraisal of the AE was terminated in 2022 following withdrawal of data by the manufacturer.8 However, decision-analytic modeling has suggested that the AE may be cost-effective in certain high-risk patient subgroups (eg, ICD recipients), accounting for the current unit cost of £800 ($1000).9,10 The existing PADIT risk score (Prior procedure, Age, Depressed renal function, Immunocompromised, Type of procedure) has been proposed as a gatekeeper strategy for AE use; however, although the discriminative power of this score has been validated in a large US registry, prognostic performance has been found inferior to other risk scores in European populations.11–15
The present study investigated the factors associated with infection for all patients with transvenous CIEDs with a view to, first, validating the PADIT risk score components in a large UK cohort and, subsequently, incorporating any additional, significant covariates into a novel risk score. The primary hypothesis was that this novel risk score may provide incremental prognostic data over and above those derived from PADIT, and hence could be used to direct more cost-effective AE use across the United Kingdom and broader CIED populations.
METHODS
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.
Patient Populations
For all patient cohorts, consecutive patients undergoing de novo implants, generator changes, and lead interventions for transvenous permanent pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization therapy devices were identified from secured registries. Lead extractions performed for infected devices were excluded. No AEs were included in this analysis.
For the derivation cohort, consecutive procedures took place at St. Bartholomew’s Hospital (SBH) from 2015 to 2019. For validation, additional data were extracted from 2 large tertiary UK cardiac centers. First, a standard-risk validation cohort combined consecutive patients at Royal Papworth Hospital from 2018 to 2019 with distinct, consecutive patients at SBH (2019–2020).
Globally, as the prevalence of complex CIED implantation and reintervention has increased, so too has the incidence of infection.16 As such, to examine the scores’ performance under high-risk conditions, a second external validation cohort was composed with an event rate of 2%. For this high-risk group, consecutive patients with CIED infection from 2014 to 2018 at St. Thomas’ Hospital were identified and combined with distinct, consecutive patients at SBH (2020–2021) with PADIT scores of ≥1. All included patients completed 12 months’ follow-up.
Procedures
Device procedures were performed in either a catheter laboratory or, in cases of high-risk lead extraction warranting standby surgical cover, in a hybrid operating theater. During the study period, patients anticoagulated for atrial fibrillation had their oral anticoagulation medications interrupted for 24 hours before the procedure. Those patients on vitamin K antagonists for a history of thromboembolism or mechanical heart valve underwent their procedures on uninterrupted anticoagulation, provided their INR was within therapeutic range (INR range, 2–3.5). No heparin bridging was used, and those inpatients prescribed heparin for prophylaxis of venous thromboembolism had this treatment withheld the evening before the procedure. Antiplatelet therapy was withheld for 5 days unless prescribed within a year of percutaneous coronary intervention or stroke. All patients received a bolus of intravenous antibiotics within 2 hours of the procedure: at SBH, patients received gentamicin 5 mg/kg (maximum dose, 450 mg) plus either flucloxacillin 1 g or, in patients with penicillin allergy or a positive or unknown methicillin-resistant staphylococcus aureus status, teicoplanin 6 mg/kg rounded to the nearest 100 mg. Patients with both penicillin and teicoplanin allergy received either a cephalosporin or vancomycin depending on the nature of the allergic reaction. At Royal Papworth Hospital, patients received gentamicin 2 mg/kg (maximum dose, 240 mg) plus either flucloxacillin 1 g or, in patients with penicillin allergy, teicoplanin 10 mg/kg. At St. Thomas’ Hospital, patients received 2 g of Flucloxacillin or 6 mg/kg of Teicoplanin if allergic to penicillin. Double gloving was mandatory during draping, with the outer gloves removed before skin incision, and the skin was prepared with chlorhexidine scrub and a 3M Ioban antimicrobial skin barrier. Local anesthetic was administered in the form of 1% lignocaine. For de novo implants, electrocautery was delivered via Pfizer ValleyLab Force FX electrosurgical generator with cut and coagulation powers set at 40 W. For reinterventions, Medtronic’s AEX generator with PlasmaBlade was used on cut and coagulation setting 5 to 6. Pocket washing was not performed routinely, however, at the operators’ discretion, intrapocket Videne antiseptic solution was administered during reinterventions with long procedure times. All lead collars were secured with Ethibond, and wounds were closed with layers of Polydioxanone, Vicryl, Monocryl, or a combination of these sutures. 3M Steri-Strips and a Softpore adhesive dressing were affixed to the skin surface, and a pressure dressing applied according to operator preference. No postprocedural oral antibiotics were prescribed in this study. Patients were advised to keep their wounds covered and dry for 7 days; this was extended to 10 days in those with a history of diabetes. All patients received follow-up—including wound inspection—at 1 month post-implant via a dedicated device clinic, and were reviewed subsequently at 12 months, or sooner if clinically indicated.
Outcomes
CIED infection was defined as hospital admission for device pocket or systemic infection within 12 months of a procedure.
Statistical analysis
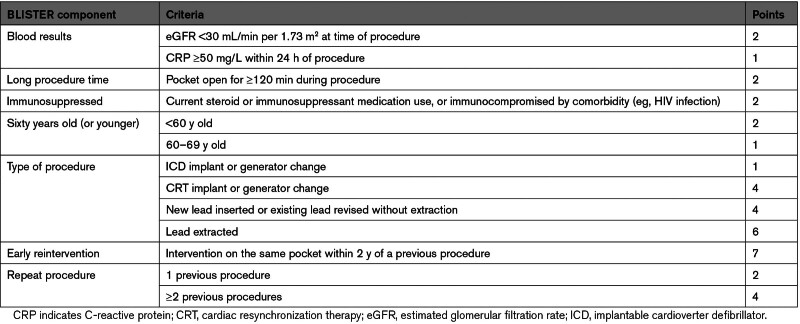
Statistical analysis was performed using R. The Shapiro-Wilk test discerned whether data were normally distributed. Categorical group variables were compared using a Z test for differences of proportion. Continuous variables were analyzed using 2-tailed unpaired t tests for normally distributed data or the Mann-Whitney U test for nonnormally distributed data. Group outcomes were compared using Fisher exact test. Univariate Cox proportional hazards analysis for the prediction of CIED infection was performed for patients’ baseline characteristics, risk factors, and procedural variables. The proportional hazards assumption was tested according to the relationship between scaled Schoenfeld residuals with time. Stepdown multivariate analysis (R package: My.stepwise) was performed subsequently including all univariate factors with P<0.25; a variance inflating factor was generated to assess for multicollinearity with a cutoff of 2.5 set for categorical variables and 10 for continuous variables. For parameters with multiple subcategories (eg, age range), multivariate analyses were repeated with a fixed reference but different subcategories applied during each iteration, with the collective final results of these models presented. An expanded PADIT score (BLISTER [Blood results, Long procedure time, Immunosuppressed, Sixty years old (or younger), Type of procedure, Early re-intervention, Repeat procedure]) was calculated based on these results by assigning weighted points to β coefficients as per Schneeweiss’ method (see Supplemental Material).17 Missing data were accounted for using regression imputation (R package: MICE). Time-dependent receiver operating characteristics curves (R package: timeROC) were calculated with prognostic performance at 12 months assessed according to differences in the area under the curve (AUC) by DeLong test.
Cost-utility analysis was performed in Microsoft Excel. Expenses were calculated from the present study cohort, including the exact cost of replacement device components in the United Kingdom, NICE tariffs for extraction and hospital bed days, and antibiotic treatment according to the British National Formulary (see Supplemental Material). Quality adjusted life year (QALY) data were taken from NICE TA 314, NICE TA 324, and other established economic analyses including post hoc analysis of the WRAP-IT trial for UK patients.10,18,19 A decision-analytic model (Figure S1) was constructed incorporating 8 possible disease states for the 12 months following a CIED procedure, and the cost utility of assigning AEs to patients according to different PADIT and BLISTER score thresholds was evaluated. The probability of device infection was based on the present study’s derivation and standard-risk validation cohorts (n=10 237, all-comers probability of infection: 0.0081), and the estimated effect size of the AE was pooled from studies included in 3 meta-analyses (Mantel-Haenszel pooled odds ratio, 0.35 [95% CI, 0.14–0.86; I2=62%).20–22 Model branches were mutually exclusive, and while the initial probability of CIED infection was calculated from the present study cohort, to promote model generalizability all subsequent probabilities were imputed from consensus in the literature (eg, probability of death if CIED infection managed without extraction, 0.422).10,23–28 Probability inputs are provided in the Supplemental Material. To account for a 12-month time-horizon in those patients undergoing CIED procedures without subsequent infection (disease state A), an annualized death rate of 5.9% was extrapolated from the standard-of-care arm in the WRAP-IT trial. Conservative management of device infection (disease state D) constituted an inpatient stay of 6 weeks for antibiotic treatment. A utility decrement of 0.1 was applied upon diagnosis of CIED infection for all device types.29 A cost per QALY gained was calculated at each risk score threshold according to whole-cohort QALY increment and the associated cost differences versus the standard of care (ie, preprocedural antibiotics and an AE versus preprocedural antibiotics only). For probabilistic sensitivity analysis, risk and transition probabilities varied according to a beta distribution, the AE efficacy varied according to a log-normal distribution, and costs varied according to a gamma distribution.30 The model results presented are average values following 10 000 iterations at each risk score threshold.
Normally distributed data are presented as mean ± SD and nonnormally distributed data as median (interquartile range). HRs are provided with 95% CIs.
Ethics
Following approval by the institutions’ governance leads, a multicenter collaboration was established on a secure online portal. As this was an analysis of registry data, the need for formal ethical approval was waived by each institution.
RESULTS
Derivation Cohort
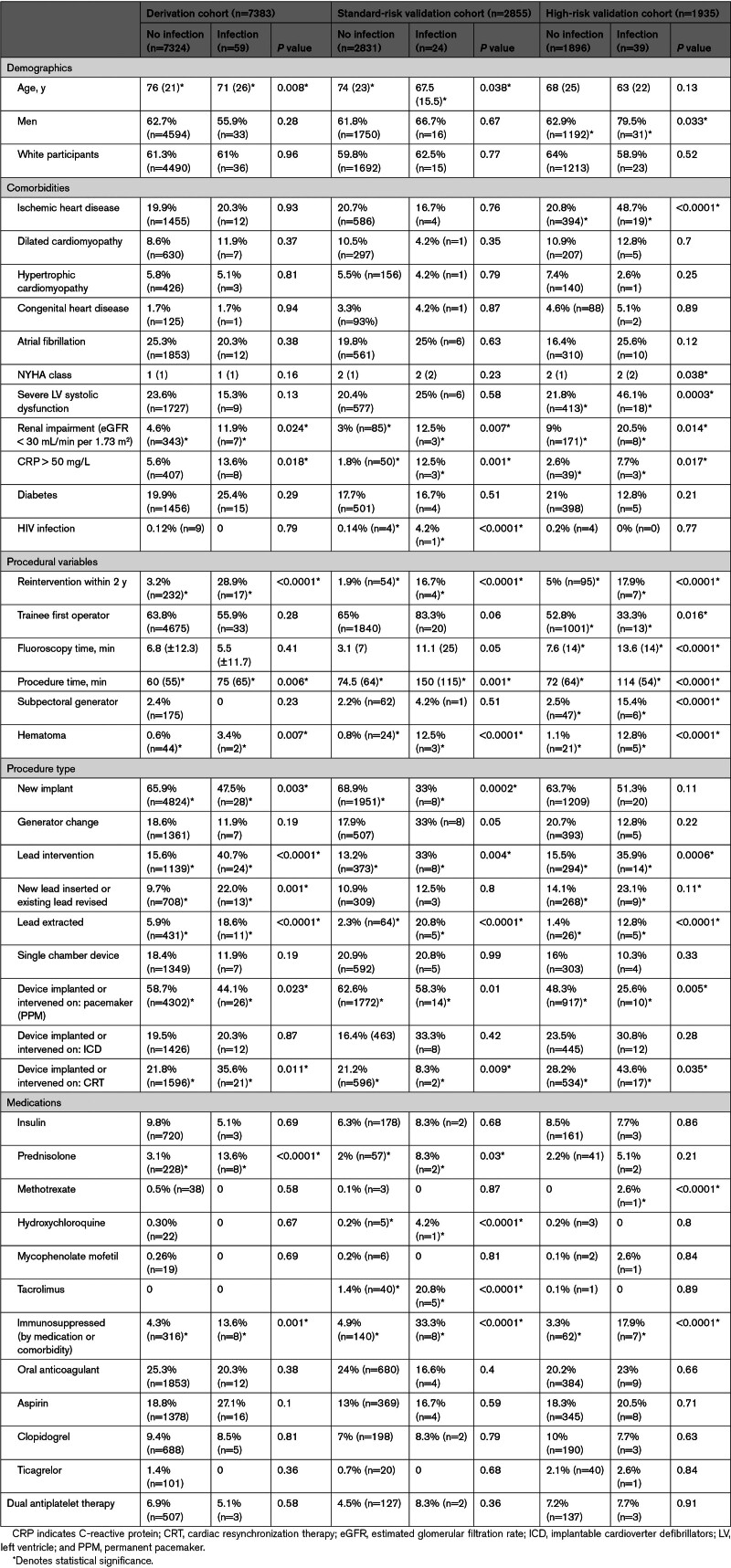
The derivation cohort included 7383 consecutive procedures at SBH between 2015 and 2019. Referral pathways consisted of direct emergency admission via the London Ambulance Service, urgent or elective referral to the institution from 11 regional hospitals, or inpatients who had developed an indication for device therapy during an admission for a primary diagnosis not related to cardiac arrhythmia (eg, following aortic valve replacement). Patient characteristics are listed in Table 1. Twenty-seven consultant physicians were listed as first operator in 36.2% of cases (n=2675), and 79 trainees or fellows in the remaining 63.8% (n=4708), performing these procedures under consultant supervision.
Table 1.
Baseline Demographics
Within 12 months, CIED infection was diagnosed in 59 individuals (incidence, 0.8%). All 59 patients with CIED infection were admitted to SBH for complete device extraction; the median hospital stay was 18 days, and the average overall cost of treatment was £18 483 (±15 139). Complete device extraction was achieved in all cases, with no associated deaths within 30 days of the procedure.
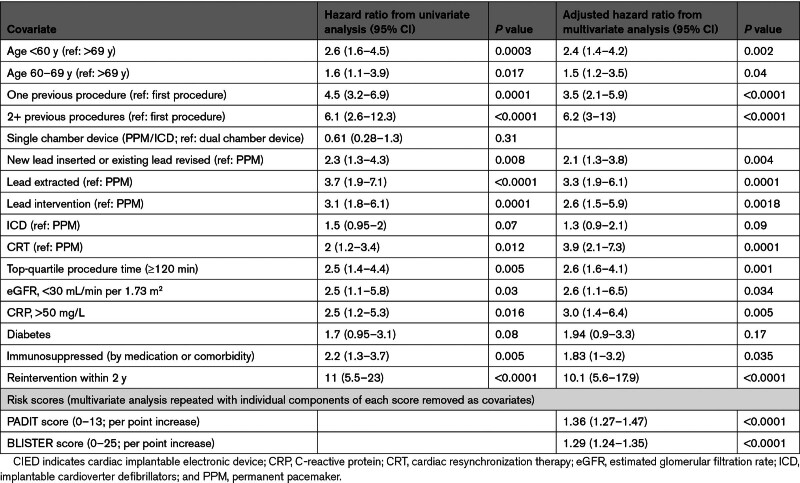
Multivariate analysis (Table 2) suggested that the components of the existing PADIT score were powerful independent predictors of infection, and 4 additional covariates (lead extraction, raised CRP (C-reactive protein), reintervention with 2 years, and top-quartile procedure duration) were incorporated into the proposed BLISTER score (Table 3). The model C-statistic was 0.78 (0.71–0.85).
Table 2.
Univariate and Final Multivariate Analysis Predicting CIED Infection at 12 Months
Table 3.
Final Proposed BLISTER Score
Validation Cohorts
The standard-risk validation cohort included 2854 consecutive procedures (2509 from SBH (88%) and 345 (12%) from Royal Papworth Hospital). CIED infection within 12 months occurred in 24 patients (event rate: 0.8%). All 24 patients underwent complete CIED extraction. The average cost of treatment for CIED infection was £20 311 (±13 684). There were no associated deaths.
The high-risk validation cohort (PADIT score, ≥1) included 1935 consecutive procedures from SBH and 26 consecutive cases of CIED infection from St. Thomas’ Hospital, with 39 cases of infection overall (n=1961, event rate: 2.0%). Two patients (5%) underwent conservative management of their CIED infection, and 1 patient (2.5%) died within 30 days of their extraction procedure. The average cost of treatment for CIED infection was £25 253 (±19 314).
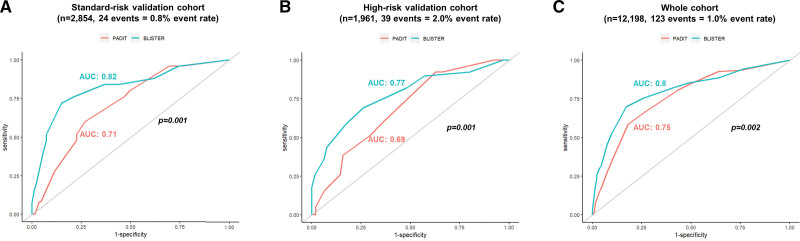
For score validation, comparative time-dependent AUC analysis at 12 months demonstrated that BLISTER was superior to PADIT in the standard-risk (AUC, 0.82 versus 0.71; P=0.001) and high-risk (AUC, 0.77 versus 0.69; P=0.001) validation cohorts, and across all patients in the derivation and validation cohorts combined (n=12 198, event rate: 1%, AUC, 0.8 versus 0.75; P=0.002; Figure 1).
Figure 1.
Time-dependent receiver operating characteristic (ROC) curves for the PADIT and BLISTER scores in diagnosing cardiac implantable electronic device (CIED) infection at 12 months. A, Standard-risk validation cohort. B, High-risk validation cohort. C, All patients from both validation cohorts and the derivation cohort.
Cost-Utility Model Results
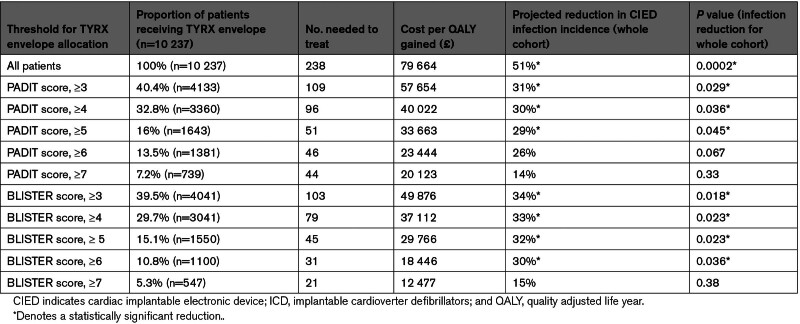
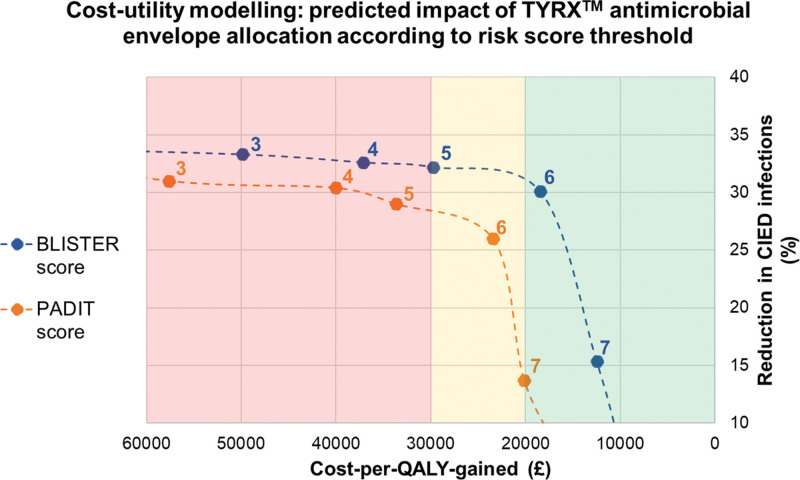
Model outcomes are provided in Table 4. For the PADIT score, assigning AEs to patients with score of ≥6 (13.5% of cohort) predicted a nonsignificant reduction in infection incidence (relative risk reduction, 26%; P=0.067) with a cost per QALY gained of £23 444. For the BLISTER score, the optimum cutoff was again a score of ≥ 6 (10.8% of cohort), predicting a significant reduction in infection (relative risk reduction, 30%; P=0.036) with a cost per QALY gained of £18 446 (Figure 2). Accordingly, when analyzed as binary factors across all 3 cohorts, PADIT and BLISTER scores of ≥ 6 were powerful predictors of CIED infection (Figure 3).
Table 4.
Results of Cost-Utility Modeling
Figure 2.
Trade-off plot demonstrating cost per quality adjusted life year (QALY) gained vs relative reduction in cardiac implantable electronic device (CIED) infections (%) for the whole cohort, with TYRX antimicrobial envelopes allocated according to different BLISTER and PADIT score thresholds.
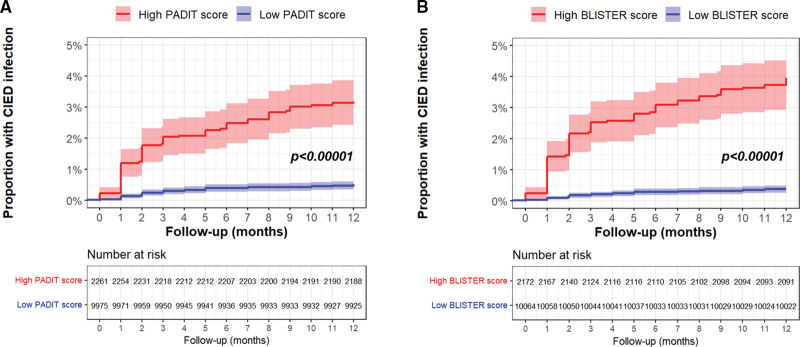
Figure 3.
Cumulative event plots for cardiac implantable electronic device (CIED) infection according to high or low PADIT and BLISTER scores [Blood results, Long procedure time, Immunosuppressed, Sixty years old (or younger), Type of procedure, Early re-intervention, Repeat procedure]. Panel A: impact of high (≥6) or low (<6) PADIT score. Panel B: impact of high (≥6) or low (<6) BLISTER score. Cumulative event plots for cardiac implantable electronic device (CIED) infection according to high or low PADIT (A) and BLISTER scores (B).
DISCUSSION
In a large, multicenter cohort including all subtypes of transvenous CIED implant, generator change, and lead intervention, the incidence of CIED infection was significant, with considerable associated health care costs. Multivariate analysis further validates the constituents of the PADIT score for predicting infection, and the incorporation of 4 additional covariates into the novel BLISTER score—lead extraction, CRP ≥50 mg/L, reintervention within 2 years, and procedure duration ≥120 minutes—conferred additional prognostic utility. Cost-utility modeling suggests that both risk scores could be used to assign the TYRX antimicrobial envelope to high-risk patients within established willingness-to-pay thresholds. A model assigning AEs to patients with a BLISTER score of ≥6 delivered superior efficacy and cost-utility versus a comparable model using a PADIT score of ≥6, despite the BLISTER model using 20% fewer AEs.
All 4 of the additional BLISTER score covariates have been associated with an increased risk of CIED infection in prior analyses.31 By analyzing lead interventions as distinct procedural subtypes, the present study found that lead extraction with lead upgrade or reimplantation confers the highest risk of infection among all procedures (adjusted hazard ratio [HR] versus pacemaker implant, 3.3 [95% CI, 1.9–6.1]; P<0.0001). There were no infected devices included in this study’s cohorts; hence, all lead extractions were performed as part of device upgrade, or to address noninfective lead integrity or veno-occlusive complications. Although these data may, therefore, suggest that, where possible, abandoning leads may be preferable to extraction for mitigating the risk of infection within 1 year, there are compelling observational data demonstrating that this strategy significantly increases the risk of complications (including infection) in the long term. Although there are usually convincing indications for CIED extraction, this approach needs to be carefully considered in clinical risk-benefit decision-making.32,33
Long procedure duration (skin-to-skin time of ≥120 minutes) was associated with CIED infection independent of procedure type (adjusted HR, 2.6 [95% CI, 1.6–4.1, P=0.001). This finding is in keeping with a meta-analysis of 60 studies by Polyzos et al, 31 who found that procedure duration correlated with infection risk, and this relationship was also corroborated in post hoc analysis of the PADIT trial (procedure duration >1 hour: OR, 1.91 [95% CI, 1.41–2.57], P<0.001), although this factor was not included in the final PADIT score. Although including this variable in the BLISTER score introduces the possibility of a small number of patients crossing the risk threshold for antimicrobial envelope use during the procedure itself, the authors suggest that the logistical challenges of implanting an unanticipated AE are outweighed by the potential benefits of protection from serious CIED infection.
Raised CRP at the time of CIED implant has a known association with infection, and in the present study 510 patients (4.2%) underwent procedures with a CRP of >50 mg/L measured within the previous 24 hours.34 This biomarker was independently associated with subsequent CIED infection (adjusted HR, 3.0 [95% CI, 1.4–6.4]; P=0.005). The subgroup was comprised of a combination of postsurgical inpatients requiring urgent pacemaker insertion (in whom permanent pacing was preferred), or direct admissions via the London Ambulance Service in whom devices were implanted emergently before the availability of blood test results. These data suggest that, whether available, CRP should be incorporated into patient risk assessment and, where possible, any source of infection should be identified and treated before a definitive CIED is implanted.
Previous procedures are well established as a risk factor for infection; in addition, the present study demonstrated that a reintervention performed within 2 years was a particularly powerful independent predictor of adverse outcomes (adjusted HR, 10.1 [95% CI, 5.6–17.9]; P<0.0001). Evidence for the association between early reintervention and CIED infection has been published previously; however, data vary on the definition and significance of the term early. Klug et al35 found a 15-fold increase in infection risk for patients undergoing reintervention during their index admission (eg, to reposition an early lead displacement). By contrast, the PADIT investigators examined the impact of reintervention within 1 month of a procedure and found no association with infection, whereas reintervention beyond 1 month predicted infection (OR, 2.45 [95% CI, 1.76–3.43], but was not included in the final PADIT risk score. In the present study, other temporal relationships were explored, for example, reintervention within 1 year (adjusted HR, 6.9 [95% CI, 3.7–17.4]; P<0.001) or within 5 years (adjusted HR, 2.9 [95% CI, 0.8–10.7]; P=0.09); however, a 2-year cutoff was found to confer maximum prognostic significance. As such, this parameter alone assigns 7 risk points in the proposed BLISTER score, and our cost-utility analysis suggests that the AE would be warranted in all patients undergoing a reintervention within 2 years of a prior procedure (n=409, 3.3%). The authors propose that this is a key factor driving the improved prognostic utility of BLISTER versus the PADIT score.
As the occurrence of hematoma cannot always be predicted before closure of the device pocket (and hence AE implantation), we did not include this factor in the BLISTER score. Nevertheless, the finding that hematomas conferred nearly a 4-fold risk of infection emphasizes the importance of good surgical technique, and measures to improve hemostasis in the CIED population are essential.
Several other economic analyses have modeled the cost utility of the AE for preventing device infection, hospitalization, and patient mortality. In a high-risk cohort, Kay at al.10 estimated a number needed to treat of 36 to prevent CIED infection, which is similar to the present study findings. However, the authors also predicted a cost per QALY gained of £46 548 for high-risk patients with pacemakers, with evidence of a cost-saving (ie, dominant) effect in those with implantable cardioverter defibrillators and CRT-Ds. Although the present study does suggest the AE to be a cost-effective treatment versus standard-of-care antibiotics in high-risk patients, it did not find the envelope to be dominant at any risk threshold. This may reflect the differences in AE effect size used between the 2 studies, with Kay et al imputing a relative risk of 0.163 versus standard of care based on observational studies published before the WRAP-IT trial. Boriani et al.19 analyzed the UK patients enrolled in the WRAP-IT study, predicting a cost per QALY gained for the AE within the NICE cost-utility threshold (ie, <£30 000) for all devices in patients with PADIT score of ≥6, and again found that the envelope became dominant in certain subgroups (eg, immunosuppressed patients with high-energy devices). Although the present analysis concurs with a PADIT score threshold of ≥6 to support cost-effective AE allocation, the more favorable results demonstrated in the Boriani analysis likely reflect the assumption that the benefits of the AE are sustained over a lifetime horizon.
Limitations
As a retrospective analysis, this study cannot demonstrate causation, and is subject to selection bias. Likewise, although consecutive patients were selected from 3 tertiary centers, the majority were from a single center (SBH). Although this may limit generalizability to other populations, the SBH data include procedures from over 100 operators and an ethnically diverse catchment population of >4 million people; hence, we suggest the cohort is sufficiently heterogeneous to confer external validity. Data completeness exceeded 90% for all parameters other than CRP (<50%); in this case, regression imputation was required, which generated a distribution of values with a strong positive skew, similar to that seen in patients with available CRP measurements. An alternative multivariate analysis performed imputing the median CRP yielded similar β coefficients. External validation of the BLISTER score demonstrated superior discriminative performance versus PADIT; however, the PADIT score was derived from a randomized cohort with inherently different levels of risk, hence, a divergence in the utility of the 2 scores may be an expected finding in a real-world population. Despite this, the fact that this divergence persisted in both standard and high-risk validation cohorts supports the generalizability of the novel score. A baseline infection rate of 0.8% was used to inform cost-utility analysis; this was calculated from an all-comers, real-world population, but nevertheless is lower than that reported in comparable registries. It is plausible that the AE is less effective in lower-risk populations and hence the present study’s cost-utility results may be overestimated. The pooled odds ratio for the antimicrobial envelope included studies with different follow-up durations; however, this calculation was heavily weighted towards the WRAP-IT trial data, which examined the same temporal end point as the BLISTER score (12 months). Although costs were summated from real-world expenses, the cost of CIED components varies broadly between manufacturers and implanting centers; hence, the present study’s cost-utility projections may not necessarily apply to other patient groups. Furthermore, the present study incorporates all costs associated with inpatient treatment of CIED infection, but does not include supplementary expenses incurred following hospital discharge (such as rehabilitation). The time-horizon used in this study’s analysis was 12 months; it is possible that the benefits of an AE may extend to additional QALY gain beyond this time period, which may have further improved cost-utility estimates. Finally, additional comorbidities that are known to influence CIED infection, such as chronic obstructive pulmonary disease, were not included in our analyzed demographics.31
Conclusions
This multicenter analysis validates the PADIT score in a large, UK population, and presents and validates the novel BLISTER score as a useful tool for risk stratification in patients with CIEDs. Economic modeling—informed by real-world costs and infection risk—suggests that risk score thresholds may facilitate individualized, cost-effective TYRX envelope (AE) allocation across large populations. A BLISTER score cutoff of ≥6 was a particularly useful prognostic marker, and incorporates key high-risk subgroups in their entirety, including patients undergoing CRT generator change, lead extraction, or reintervention within 2 years. At this level of patient risk, the number needed to treat with an AE to prevent a CIED infection was estimated at 31.
Our institutions have adopted the BLISTER score into routine clinical practice; prospective validation is ongoing. A free online calculator is available to facilitate point-of-care decision-making (https://qxmd.com/calculate/calculator_876/the-blister-score-for-cied-infection).36
ARTICLE INFORMATION
Sources of Funding
P.L. is supported by University College London Hospital Biomedical Research Center and the National Institute for Health and Care Research Barts Biomedical Research center.
Disclosures
None.
Supplemental Material
Tables S1–S9
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AE
- antimicrobial envelope
- AUC
- area under the curve
- CIED
- cardiac implantable electronic device
- CRP
- C-reactive protein
- CRT
- cardiac resynchronization therapy
- HR
- hazard ratio
- NICE
- National Institute for Health and Care Excellence
- QALY
- quality adjusted life year
- SBH
- St. Bartholomew’s Hospital
For Sources of Funding and Disclosures, see page 158.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.123.012446.
Contributor Information
Edd Maclean, Email: e.maclean@nhs.net.
Karishma Mahtani, Email: karishma.mahtani1@nhs.net.
Shohreh Honarbakhsh, Email: sherry0508@doctors.org.uk.
Charles Butcher, Email: charles.butcher2@nhs.net.
Nikhil Ahluwalia, Email: nikhil.ahluwalia@nhs.net.
Adam S.C. Dennis, Email: adam.dennis@nhs.net.
Antonio Creta, Email: a.creta@nhs.net.
Malcolm Finlay, Email: malcolm.finlay1@nhs.net.
Mark Elliott, Email: markkenneth.elliott@nhs.net.
Vishal Mehta, Email: vishal.mehta@kcl.ac.uk.
Omar Shaikh, Email: o.shaikh@nhs.net.
Chizute Ogbedeh, Email: chizute.ogbedeh@nhs.net.
Vasu Gautam, Email: vg340@cam.ac.uk.
Pier D. Lambiase, Email: p.lambiase@ucl.ac.uk.
Richard J. Schilling, Email: Richard.schilling@nhs.net.
Mark J. Earley, Email: mark.earley1@nhs.net.
Philip Moore, Email: philip.moore3@nhs.net.
Amal Muthumala, Email: a.muthumala@nhs.net.
Ross J. Hunter, Email: ross.hunter3@nhs.net.
Christopher A. Rinaldi, Email: aldo.rinaldi@kcl.ac.uk.
Jonathan Behar, Email: jonathanbehar@gmail.com.
Claire Martin, Email: claire.martin22@nhs.net.
REFERENCES
- 1.Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR, Krahn AD, Schloss EJ, Gallastegui JL, Pickett RA, et al. Cost-effectiveness of an antibacterial envelope for cardiac implantable electronic device infection prevention in the US healthcare system from the WRAP-IT Trial. Circ Arrhythm Electrophysiol. 2020;13:1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR, Love JC, Augostini R, Faerestrand S, Wiggins SS, et al. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. 2020;13:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M, Cai C, Vaibhav V, Sohail MR, Hayes DL, Hodge DO, Tian Y, Asirvatham R, Cochuyt JJ, Huang C, et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. JACC Clin Electrophysiol. 2019;5:1071–1080. doi: 10.1016/j.jacep.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 4.Rennert-May E, Chew D, Lu S, Chu A, Kuriachan V, Somayaji R. Epidemiology of cardiac implantable electronic device infections in the United States: a population-based cohort study. Heart Rhythm. 2020;17:1125–1131. doi: 10.1016/j.hrthm.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 5.Banks H, Torbica A, Valzania C, Varabyova Y, Prevolnik Rupel V, Taylor RS, Hunger T, Walker S, Boriani G, Fattore G; MedtecHTA Group. Five year trends (2008-2012) in cardiac implantable electrical device utilization in five European nations: a case study in cross-country comparisons using administrative databases. Europace. 2018;20:643–653. doi: 10.1093/europace/eux123 [DOI] [PubMed] [Google Scholar]
- 6.Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG, Poole J, Boriani G, Costa R, Deharo JC, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020;41:2012–2032. doi: 10.1093/eurheartj/ehaa010 [DOI] [PubMed] [Google Scholar]
- 7.Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E, Gallastegui J, Pickett RA, Evonich R, Philippon F, et al. ; WRAP-IT Investigators. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;380:1895–1905. doi: 10.1056/NEJMoa1901111 [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. TYRX Absorbable Antibacterial Envelope for preventing infection from cardiac implantable electronic devices [ID1440]. 2022. https://www.nice.org.uk/guidance/indevelopment/gid-ta10370
- 9.Burnhope E, Rodriguez-Guadarrama Y, Waring M, Guilder A, Malhotra B, Razavi R, Rinaldi CA, Pennington M, Carr-White G. Economic impact of introducing TYRX amongst patients with heart failure and reduced ejection fraction undergoing implanted cardiac device procedures: a retrospective model based cost analysis. J Med Econ. 2019;22:464–470. doi: 10.1080/13696998.2019.1581621 [DOI] [PubMed] [Google Scholar]
- 10.Kay G, Eby EL, Brown B, Lyon J, Eggington S, Kumar G, Fenwick E, Sohail MR, Wright DJ. Cost-effectiveness of TYRX absorbable antibacterial envelope for prevention of cardiovascular implantable electronic device infection. J Med Econ. 2018;21:294–300. doi: 10.1080/13696998.2017.1409227 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed FZ, Blomström-Lundqvist C, Bloom H, Cooper C, Ellis C, Goette A, Greenspon AJ, Love CJ, Johansen JB, Philippon F, et al. Use of healthcare claims to validate the prevention of arrhythmia device infection trial cardiac implantable electronic device infection risk score. Europace. 2021;23:1446–1455. doi: 10.1093/europace/euab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sławek-Szmyt S, Araszkiewicz A, Grygier M, Szmyt K, Chmielewska-Michalak L, Seniuk W, Waśniewski M, Smukowski T, Lesiak M, Mitkowski P. Predictors of long-term infections after cardiac implantable electronic device surgery: Utility of novel PADIT and PACE DRAP scores. Circ J. 2020;84:1754–1763. doi: 10.1253/circj.CJ-20-0305 [DOI] [PubMed] [Google Scholar]
- 13.Malagù M, Donazzan L, Capanni A, Sirugo P, Rapezzi C, Bertini M. Risk scores for cardiac implantable electronic device infection: which one to believe in? J Clin Med. 2022;11:6556. doi: 10.3390/jcm11216556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhry U, Borgquist R, Smith JG, Mörtsell D. Efficacy of the antibacterial envelope to prevent cardiac implantable electronic device infection in a high-risk population. Europace. 2022;24:1973–1980. doi: 10.1093/europace/euac119 [DOI] [PubMed] [Google Scholar]
- 15.Boriani G, Proietti M, Bertini M, Diemberger I, Palmisano P, Baccarini S, Biscione F, Bottoni N, Ciccaglioni A, Monte AD, et al. Incidence and predictors of infections and all-cause death in patients with cardiac implantable electronic devices: the Italian Nationwide RI-AIAC Registry. J Pers Med. 2022;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han HC, Hawkins NM, Pearman CM, Birnie DH, Krahn AD. Epidemiology of cardiac implantable electronic device infections: incidence and risk factors. Europace. 2021;23:iv3–iv10. doi: 10.1093/europace/euab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38:1103–1120. doi: 10.1111/1475-6773.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure. NICE Guidel. 2014; Technology Appraisal 314. https://www.nice.org.uk/guidance/ta314 [Google Scholar]
- 19.Boriani G, Kennergren C, Tarakji KG, Wright DJ, Ahmed FZ, McComb JM, Goette A, Blum T, Biffi M, Green M, et al. Cost-effectiveness analyses of an absorbable antibacterial envelope for use in patients at increased risk of cardiac implantable electronic device infection in Germany, Italy, and England. Value Health. 2021;24:930–938. doi: 10.1016/j.jval.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 20.Pranata R, Tondas AE, Vania R, Yuniadi Y. Antibiotic envelope is associated with reduction in cardiac implantable electronic devices infections especially for high-power device—Systematic review and meta-analysis. J Arrhythm. 2020;36:166–173. doi: 10.1002/joa3.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Doshi R, Shariff M. Role of antibiotic envelopes in preventing cardiac implantable electronic device infection: a meta-analysis of 14 859 procedures. J Arrhythm. 2020;36:176–179. doi: 10.1002/joa3.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullah W, Nadeem N, Haq S, Thelmo FL, Abdullah HM, Haas DC. Efficacy of antibacterial envelope in prevention of cardiovascular implantable electronic device infections in high-risk patients: a systematic review and meta-analysis. Int J Cardiol. 2020;315:51–56. doi: 10.1016/j.ijcard.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 23.Sandoe JAT, Barlow G, Chambers JB, Gammage M, Guleri A, Howard P, Olson E, Perry JD, Prendergast BD, Spry MJ, et al. ; British Society for Antimicrobial Chemotherapy. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. J Antimicrob Chemother. 2015;70:325–359. doi: 10.1093/jac/dku383 [DOI] [PubMed] [Google Scholar]
- 24.Sohail MR, Uslan DZ, Khan AH, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, Jenkins SM, Baddour LM. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc. 2008;83:46–53. doi: 10.4065/83.1.46 [DOI] [PubMed] [Google Scholar]
- 25.Shariff N, Eby E, Adelstein E, Jain S, Shalaby A, Saba S, Wang NC, Schwartzman D. Health and economic outcomes associated with use of an antimicrobial envelope as a standard of care for cardiac implantable electronic device implantation. J Cardiovasc Electrophysiol. 2015;26:783–789. doi: 10.1111/jce.12684 [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Gracely EJ, Aleem SY, Kutalek SP, Vielemeyer O. Differences of mortality rates between pocket and nonpocket cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol. 2015;38:1456–1463. doi: 10.1111/pace.12748 [DOI] [PubMed] [Google Scholar]
- 27.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol. 2011;58:1001–1006. doi: 10.1016/j.jacc.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 28.Tarakji KG, Wazni OM, Harb S, Hsu A, Saliba W, Wilkoff BL. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. 2014;16:1490–1495. doi: 10.1093/europace/euu147 [DOI] [PubMed] [Google Scholar]
- 29.NICE. Dual-chamber pacemakers for symptomatic bradycardia due to sick sinus syndrome without atrioventricular block NICE-TA324. NICE Guid. 2014. https://www.nice.org.uk/guidance/ta324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briggs A. Probabilistic analysis of cost-effectiveness models: Statistical representation of parameter uncertainty. Value Health. 2005;8:1–2. doi: 10.1111/j.1524-4733.2005.08101.x [DOI] [PubMed] [Google Scholar]
- 31.Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace. 2015;17:767–777. doi: 10.1093/europace/euv053 [DOI] [PubMed] [Google Scholar]
- 32.Maytin M, Epstein LM, Henrikson CA. Lead extraction is preferred for lead revisions and system upgrades when less is more. Circ Arrhythm Electrophysiol. 2010;3:413–24; discussion 424. doi: 10.1161/CIRCEP.110.954107 [DOI] [PubMed] [Google Scholar]
- 33.Hussein AA, Tarakji KG, Martin DO, Gadre A, Fraser T, Kim A, Brunner MP, Barakat AF, Saliba WI, Kanj M, et al. Cardiac implantable electronic device infections: added complexity and suboptimal outcomes with previously abandoned leads. JACC Clin Electrophysiol. 2017;3:1–9. doi: 10.1016/j.jacep.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 34.Sławiński G, Kempa M, Lewicka E, Budrejko S, Królak T, Raczak G. Elevated C-reactive protein levels during cardiac implantations may increase the risk of early complications requiring transvenous lead removal: a preliminary report. Polish Arch Intern Med. 2018;128:138–140. [DOI] [PubMed] [Google Scholar]
- 35.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clementy J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, et al. ; PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664 [DOI] [PubMed] [Google Scholar]
- 36.QxMD: The BLISTER Score. https://qxmd.com/calculate/calculator_876/the-blister-score-for-cied-infection.
- 37.Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol. 2018;41:495–503. doi: 10.1111/pace.13300 [DOI] [PubMed] [Google Scholar]
- 38.Deckx S, Marynissen T, Rega F, Ector J, Nuyens D, Heidbuchel H, Willems R. Predictors of 30-day and 1-year mortality after transvenous lead extraction: a single-centre experience. Europace. 2014;16:1218–1225. doi: 10.1093/europace/eut410 [DOI] [PubMed] [Google Scholar]
- 39.NICE. British National Formulary (BNF). 2023.
- 40.NHS England. NHS: National Tariff Payment System. 2022. https://www.england.nhs.uk/pay-syst/national-tariff/national-tariff-payment-system/
- 41.NHS Digital. NHS Digital: Reference Costs Collection. 2017. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/reference-costs
- 42.Feldman AM, De Lissovoy G, Bristow MR, Saxon LA, De Marco T, Kass DA, Boehmer J, Singh S, Whellan DJ, Carson P, et al. Cost effectiveness of cardiac resynchronization therapy in the comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2311–2321. doi: 10.1016/j.jacc.2005.08.033 [DOI] [PubMed] [Google Scholar]
- 43.Udo EO, Van Hemel NM, Zuithoff NPA, Nijboer H, Taks W, Doevendans PA, Moons KGM. Long term quality-of-life in patients with bradycardia pacemaker implantation. Int J Cardiol. 2013;168:2159–2163. doi: 10.1016/j.ijcard.2013.01.253 [DOI] [PubMed] [Google Scholar]
- 44.Bundgaard JS, Thune JJ, Nielsen JC, Videbæk R, Haarbo J, Bruun NE, Videbæk L, Aagaard D, Korup E, Jensen G, et al. The impact of implantable cardioverter-defibrillator implantation on health-related quality of life in the DANISH trial. Europace. 2019;21:900–908. doi: 10.1093/europace/euz018 [DOI] [PubMed] [Google Scholar]
- 45.Henrikson CA, Sohail MR, Acosta H, Johnson EE, Rosenthal L, Pachulski R, Dan D, Paladino W, Khairallah FS, Gleed K, et al. Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement: results of the citadel and centurion studies. JACC Clin Electrophysiol. 2017;3:1158–1167. doi: 10.1016/j.jacep.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 46.Hassoun A, Thottacherry ED, Raja M, Scully M, Azarbal A. Retrospective comparative analysis of cardiovascular implantable electronic device infections with and without the use of antibacterial envelopes. J Hosp Infect. 2017;95:286–291. doi: 10.1016/j.jhin.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 47.Kolek MJ, Patel NJ, Clair WK, Whalen SP, Rottman JN, Kanagasundram A, Shen ST, Saavedra PJ, Estrada JC, Abraham RL, et al. Efficacy of a bio-absorbable antibacterial envelope to prevent cardiac implantable electronic device infections in high-risk subjects. J Cardiovasc Electrophysiol. 2015;26:1111–1116. doi: 10.1111/jce.12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittal S, Shaw RE, Michel K, Palekar R, Arshad A, Musat D, Preminger M, Sichrovsky T, Steinberg JS. Cardiac implantable electronic device infections: Incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm. 2014;11:595–601. doi: 10.1016/j.hrthm.2013.12.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.