Extended Data Fig. 9 |. Model.
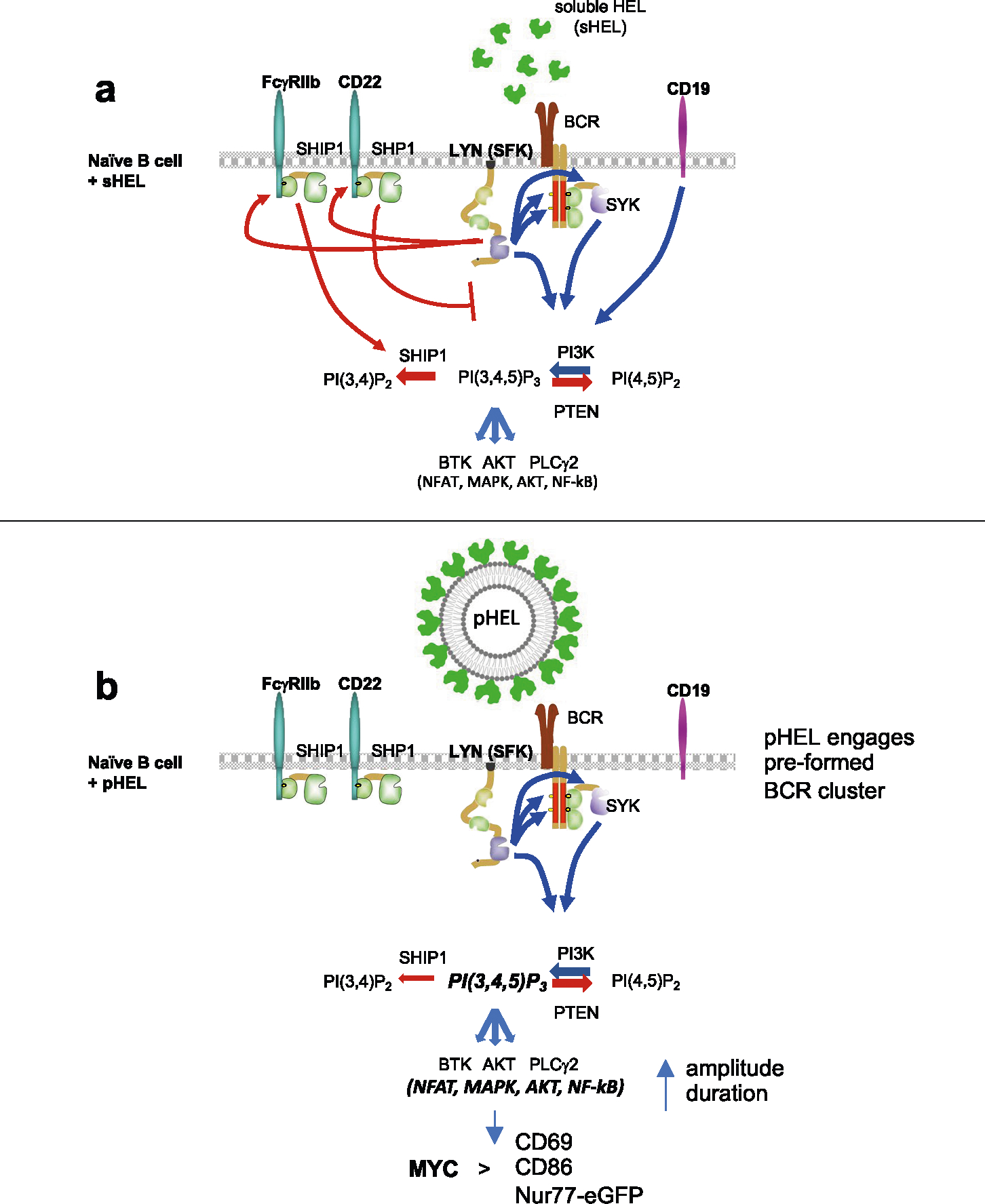
a. Signalosome assembly in naïve B cells upon BCR stimulation by soluble Ag. BCR signal transduction requires sequential action of Src family kinases (SFKs) and SYK kinase. CD19 engagement amplifies PI3K activation and production of PI(3,4,5)P3. While multiple SFKs can mediate ITAM signaling downstream of the BCR, the SFK LYN plays a non-redundant role in phosphorylating ITIM-containing inhibitory coreceptors which in turn recruit PTPases SHP1 and SHIP1 that suppress PIP3. Dynamic regulation of PIP3 at the plasma membrane controls amplitude of signaling by recruiting downstream mediators including AKT, BTK, and PLCγ2 to orchestrate transcriptional programs mediated by NFAT, NF-κB and other factors. b. SVLS with appropriately spaced epitopes robustly engage pre-existing BCR nanoclusters but evade co-inhibitory receptors and results in downstream signal amplification. SVLS do not rely upon CD19 engagement for signal amplification in vitro. In the absence of inhibitory PTPase engagement via ITIM-containing inhibitory receptors, PIP3 accumulates at the plasma membrane leading to enhanced and prolonged signalosome assembly and activity downstream of SVLS stimulation. Robust NFAT and NF-κB accumulation in the nucleus and AKT-dependent signals mimic co-stimulation and promote MYC expression, resulting in T-independent cell growth, survival, and proliferation.
