Extended Data Fig. 1 |. pHEL SVLS library and in vitro potency.
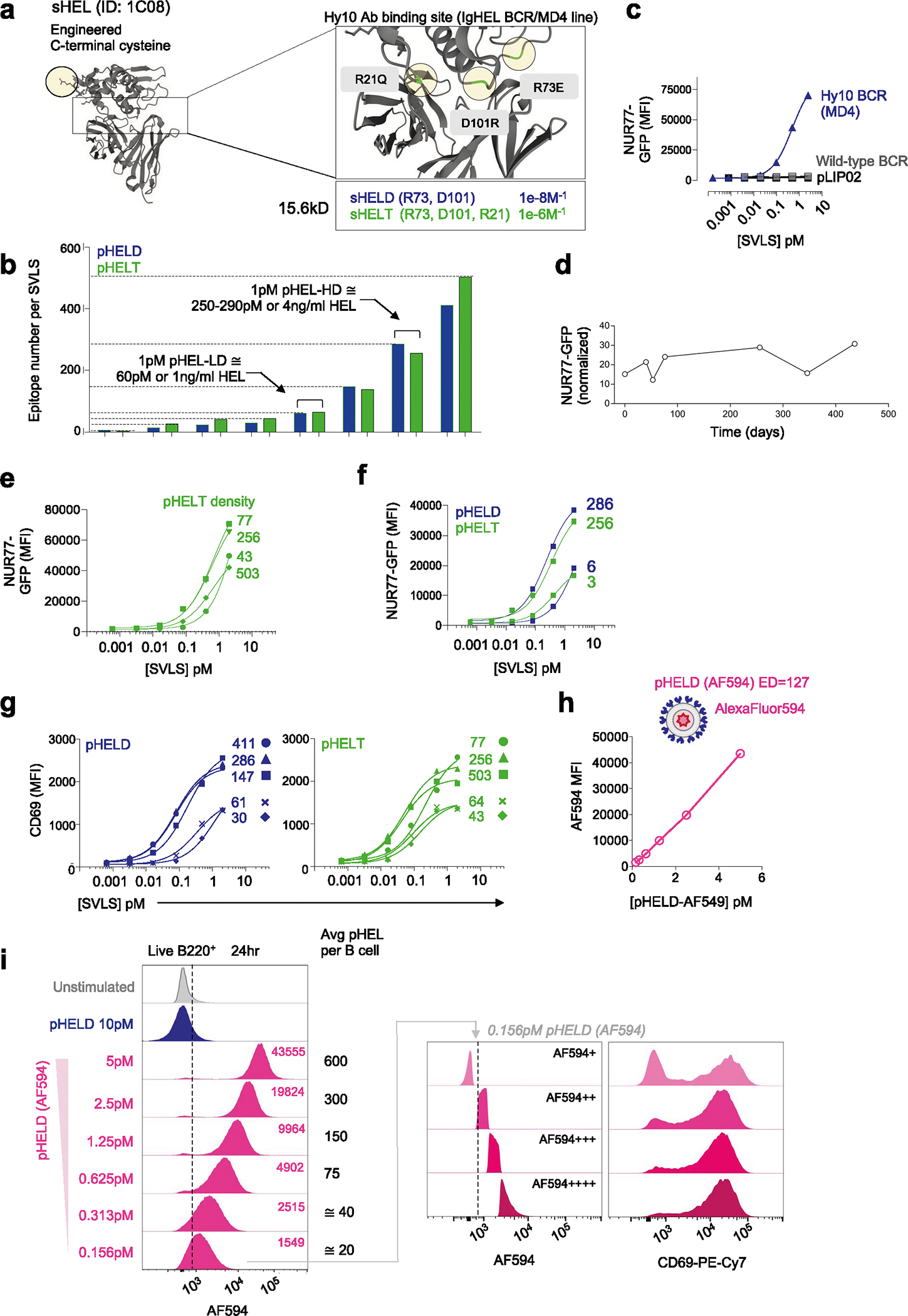
a. Hen egg lysozyme (HEL) engineered with c-terminal cysteine residue and point mutations (zoomed box) described to reduce binding affinity to Hy10 BCR38. b. Schematic of SVLS library constructed with varying densities of HELD or HELT conjugated to liposomes yielding pHELD/T particles (3–500 epitope density/particle, calculated as [HEL]/[lip] in M). Each bar represents a single pHEL preparation to illustrate range. c. Congenically-marked NUR77-GFP reporter MD4 (Hy10) mixed with wild-type splenocytes and stimulated with pHELD or naked control liposome (pLIP02) for 24 hr. GFP was analysed in B220+ cells of each gt as in Main Fig. 1c. d. NUR77-GFP expression was analysed in MD4 B cells at 24 hr post-stimulation with the same batch of pHELD at various times post-synthesis, normalized to the pLIP02 condition in each experiment. e. As in main Fig. 1e but with pHELT at varying ED. Data is from a single experiment and representative of 3 independent experiments. f. As in E, but stimulated with high or ultra-low density pHELD/T. Data is from a single experiment. g. As in E, but depicting CD69 after 24 hr with pHELD/T. Data are from a single experiment and representative of 3 independent experiments. Curves were modelled with three-parameter nonlinear regression (C, E, F, G). h, i. SVLS with HELD conjugated at ED 124 and encapsulated AF594, herein termed pHELD(AF594), mixed at serial dilutions with MD4 cells for 24 hr and analyzed by flow cytometry. H. Quantification of AF594 fluorescence in B220+ lymphocytes. I. Representative histograms (left) depict AF594 fluorescence in B220+ cells. Inset values: AF594 MFI. Average particles/B cell in culture based on concentrations of each. Right-hand histograms depict 0.156pM-stimulated B cells sub-gated according to AF594 fluorescence to represent capture of particles. Data are from a single experiment and representative of 3 independent experiments.
