Author response image 2. FKBP12/FRB-induced trapping experiments.
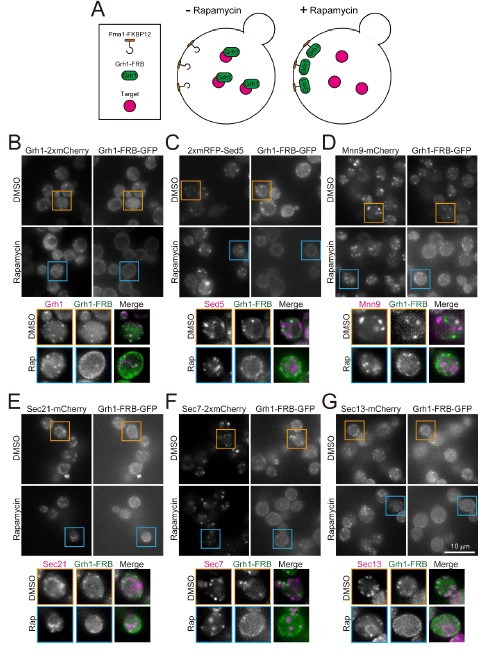
(A) The strategy of the experiments. We made rapamycin-resistant yeast strains in which FRB-GFP is fused to Grh1 (an ERGIC protein), FKBP12 is fused to Pma1 (a plasma membrane protein), and red fluorescent protein (mCherry or mRFP) is fused to a target protein. If the Grh1-FRB-GFP is recruited to the plasma membrane (where Pma1-FKBP12 is localized) together with the target protein after treatment with rapamycin, this suggests that they were in the same membrane compartment. (B) Control experiment. Upon treatment with rapamycin, Grh1-FRB-GFP was recruited to the plasma membrane, but Grh1-mCherry was not. (C-G) Sed5 (another ERGIC protein), Mnn9 (a cis-Golgi protein), Sec21 (a medial Golgi protein), Sec7 (a TGN protein), and Sec13 (an ERES protein) were also not recruited to the plasma membrane. These results suggest that, at least in this experimental system, when Grh1-FRB-GFP is captured at the plasma membrane by rapamycin treatment, the membrane compartment on which Grh1 rode does not migrate together with it.
