Abstract
Background
The impact of recent consensus definitions of cancer therapy–related cardiac dysfunction (CTRCD) from the European Society of Cardiology cardio-oncology guidelines on the reported incidence of CTRCD has not yet been assessed.
Objectives
The aim of this study was to assess the: 1) cumulative incidence; 2) point prevalence during and after adjuvant therapy; and 3) prognostic value of CTRCD as defined by different asymptomatic CTRCD guideline criteria.
Methods
The cumulative incidence and point prevalence of CTRCD were retrospectively assessed in 118 patients participating in the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial. Asymptomatic CTRCD was assessed using alternative cardiac troponin (cTn) 99th percentile upper reference limits (URLs) to define cTnT and cTnI elevation.
Results
The cumulative incidence of moderate or severe CTRCD was low (1.7%), whereas the cumulative incidence of mild asymptomatic CTRCD was higher and differed markedly according to the biomarker criteria applied, ranging from 49.2% of patients when cTnT greater than the sex-specific 99th percentile URL was used to define cTn elevation to 9.3% when sex-neutral cTnI was used. The point prevalence of CTRCD was highest at the end of anthracycline therapy (47.8%) and was driven primarily by asymptomatic cTn elevation. CTRCD during adjuvant therapy was not prognostic for CTRCD at extended follow-up of 24 months (Q1-Q3: 21-29 months) after randomization.
Conclusions
Mild asymptomatic CTRCD during adjuvant breast cancer therapy was frequent and driven mainly by cTn elevation and was not prognostic of subsequent CTRCD. The incidence of mild, asymptomatic CTRCD differed markedly depending on the cTn assay and whether sex-neutral or sex-dependent URLs were applied. (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy [PRADA]; NCT01434134)
Key Words: biomarkers, breast cancer, cardiac magnetic resonance, cardiomyopathy, guidelines, troponin
Central Illustration
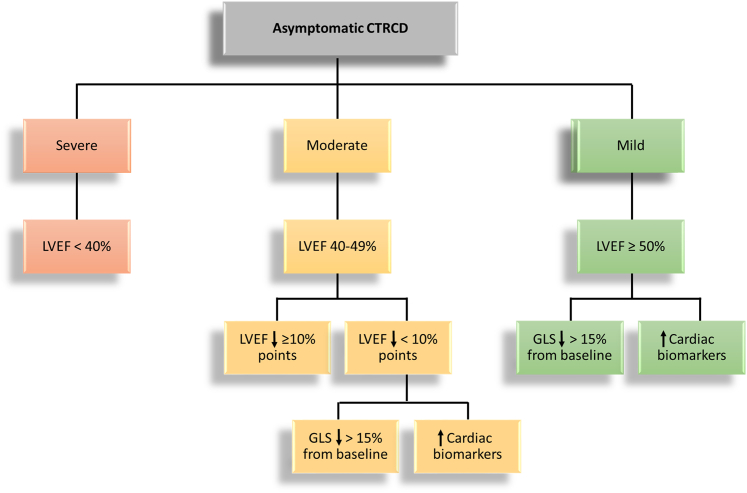
Cancer therapy with potentially cardiotoxic agents such as anthracyclines, monoclonal antibodies (trastuzumab, pertuzumab), CDK4/6 inhibitors (ribociclib, palbociclib, abemaciclib), and radiotherapy has contributed to cardiovascular disease being an important cause of morbidity and potentially fatal outcomes among breast cancer survivors.1,2 Numerous different definitions of cancer therapy–associated cardiotoxicity have been used, making it difficult to compare results across trials to determine the true incidence and optimal management strategies for different cardiovascular cardiotoxicities and cancer therapy–related cardiac dysfunction (CTRCD). Therefore, the recent consensus definitions of cardiovascular toxicities from the International Cardio-Oncology Society (IC-OS),3 adopted in the 2022 European Society of Cardiology (ESC) cardio-oncology guidelines,4 represent an important step toward a uniform understanding and agreement of what constitutes cardiac toxicity. This CTRCD definition distinguishes between symptomatic heart failure (HF) and asymptomatic cardiac dysfunction, ranging from mild to severe. Asymptomatic cases, which are far more common than symptomatic cases, are graded according to left ventricular ejection fraction (LVEF) and LVEF change, and severity is further assessed by measures of change in echocardiographic left ventricular global longitudinal strain (GLS) and circulating cardiovascular biomarkers. More specifically, a new relative decline in GLS by 15% from baseline and/or a new rise in cardiac biomarkers, defined as cardiac troponin (cTn) I or T greater than the 99th percentile upper reference limit (URL), B-type natriuretic peptide ≥35 ng/L, or amino-terminal pro–B-type natriuretic peptide (NT-proBNP) ≥125 ng/L3,4 (Figure 1). However, the incidence, prevalence, and prognostic value of CTRCD according to these new definitions have not yet been assessed. Moreover, whether the use of different biomarker criteria, for instance, cTnT vs cTnI and sex-specific vs -neutral 99th percentile cutoff values, provides different incidence and risk estimates remains unclear. Therefore, the aim of the present study was to retrospectively assess the: 1) cumulative incidence of CTRCD; 2) point prevalence of CTRCD during and after therapy; and 3) prognostic value of different asymptomatic CTRCD guideline biomarker criteria in patients receiving adjuvant anthracycline-containing chemotherapy for early breast cancer.
Figure 1.
CTRCD Definition
Asymptomatic cancer therapy–related cardiac dysfunction (CTRCD) according to the International Cardio-Oncology Society. GLS = global longitudinal strain; LVEF = left ventricular ejection fraction.
Methods
Study design and participants
Study design and procedures have previously been described in detail.5, 6, 7 The PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial was a randomized, 2 × 2 factorial, placebo-controlled, double-blind clinical trial conducted at Akershus University Hospital in Norway. The study protocol was approved by the Regional Ethics Committee of South-Eastern Norway (approval number 2010/2890), registered at ClinicalTrials.gov (NCT01434134), and was conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants provided written informed consent before any study procedures took place. Eligible patients were adult women between 18 and 70 years of age with early breast cancer scheduled for treatment with anthracyclines with or without taxanes, radiotherapy, and trastuzumab. The main inclusion criterion was Eastern Cooperative Oncology Group status 1 or 2, and the main exclusion criteria was clinically significant heart disease, impaired renal function, LVEF <50%, indication or contraindication to treatment with the study medication, and prior treatment with anthracyclines or radiotherapy. Data from the PRADA trial cannot be publicly shared because of the risk for violating privacy, as regulated by the institutional data protection officer.
Study procedures
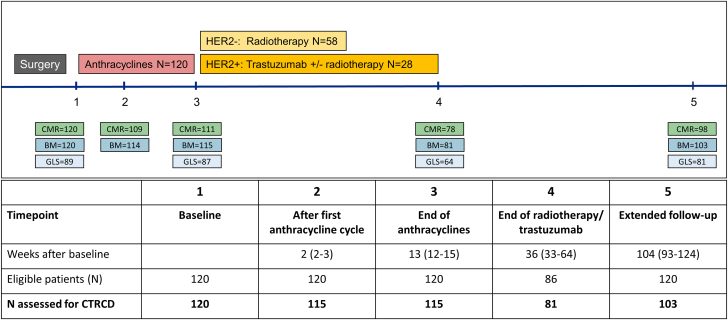
Eligible patients were randomized to 1 of 4 treatment combinations: metoprolol succinate and placebo, candesartan cilexetil and placebo, metoprolol succinate and candesartan cilexetil, and double placebo. The study was double blind. Patients were evaluated using physical examinations, blood samples, and cardiac magnetic resonance (CMR) at baseline, after the first anthracycline cycle, after the completion of anthracycline treatment, for patients who received additional treatment with radiotherapy or trastuzumab after completion of this therapy, and at an extended follow-up point of 24 months (Q1-Q3: 21-29 months) after randomization. Echocardiography was performed at the same time points, except not after the first anthracycline cycle (Figure 2).
Figure 2.
Study Timeline and Procedures
Overview of PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study timeline and available measurements at each time point. BM = biomarkers; CMR = cardiac magnetic resonance imaging; HER2 = human epidermal growth factor receptor 2; other abbreviations as in Figure 1.
CMR and echocardiography
All CMR examinations were performed using a 1.5-T magnetic resonance imaging scanner (Achieva, Philips Medical Systems). Transthoracic echocardiography was performed using a commercially available system (E9, GE Vingmed Ultrasound).
Biomarker measurements
cTnI was measured using the STAT High Sensitive Troponin-I assay (Abbott Diagnostics). cTnT was measured using a high-sensitivity assay (Troponin T hs STAT) and NT-proBNP using the proBNPII assay (Roche Diagnostics). The sex-specific 99th percentile URLs for cTnT and cTnI for women are 9.0 and 15.6 ng/L on the platforms used in this study, and the corresponding sex-neutral 99th percentile URLs are 14 and 26.2 ng/L.8 In this study, we used the recommended IC-OS cutoff value of 125 ng/L for NT-proBNP.
Additional details regarding imaging and biomarker procedures are provided in the Supplemental Appendix and in previous reports.5, 6, 7
Definition of cardiotoxicity
The occurrence of CTRCD was assessed at each time point according to the IC-OS definitions, where symptomatic CTRCD was graded according to HF symptoms ranging from mild to severe. Asymptomatic CTRCD was defined as 1) severe when there was a new LVEF reduction to <40%; 2) moderate when LVEF declined by ≥10 percentage points to an LVEF of 40% to 49% or LVEF declined by <10 percentage points to an LVEF of 40% to 49% and there was a new relative decline in GLS by >15% from baseline or new rise in cardiac biomarkers above the defined 99th percentile cutoff values; and 3) mild when LVEF was ≥50% and there was a new relative decline in GLS by >15% from baseline and/or a new rise in cardiac biomarkers. In this study elevation in cTns is defined according to both sex-specific and sex-neutral URLs for cTnI and cTnT, whereas we use the recommended IC-OS sex-neutral cutoff value for NT-proBNP.
Statistical analysis
Counts and percentages are reported for all categorical variables. Continuous variables are reported as median (Q1-Q3). Baseline characteristics are reported according to development of CTRCD during the observation period with a cTnT value greater than the sex-specific 99th percentile URL as the cTn criterion. Comparisons of groups were made using the Mann-Whitney U test for continuous variables and the chi-square or Fisher exact test for categorical variables, as appropriate. The CTRCD cumulative incidence and point prevalence at a given time point were calculated for patients with at least 1 biomarker or CMR measurement. A series of univariable logistic regression analyses were used to assess the prognostic value of a CTRCD diagnosis at the end of anthracycline therapy for CTRCD at extended follow-up and to assess the effect of randomization status on the point prevalence of CTRCD at the end of anthracycline therapy and at extended follow-up. ORs and 95% CIs were calculated. To assess the prognostic value of baseline risk factors and the effect of randomization status on the cumulative CTRCD incidence, a series of separate mixed-effects logistic regression models, including age, systolic blood pressure, epirubicin dose, trastuzumab, and study medication (metoprolol, candesartan, and placebo), were fitted for each risk factor to all available measurements from all time points. All mixed-effects logistic regression models included fixed effects for measurement time point and a random intercept. Analyses were adjusted for CTRCD criteria fulfilled at baseline. We used the Box-Tidwell procedure to assess potential deviations from the assumption of linearity between continuous variables and the logit of the ORs. The function boxTidwell in the software package car in R was used. No deviations were found. We used RStudio version 1.4.1717 and R version 3.4.4 (R Foundation for Statistical Computing) for mixed-models analyses, using glmer in the lme4 package. All other statistical analyses were performed using Stata version 17 (StataCorp).
Results
Cumulative incidence and point prevalence of CTRCD according to different criteria
Baseline characteristics and biomarker values are summarized in Table 1, which includes the 118 of 120 patients who had at least 1 valid assessment of CTRCD after the initiation of anthracyclines. In the PRADA cohort, 120 patients underwent CMR at baseline, 109 after the first cycle of anthracyclines, 111 at the completion of anthracycline therapy, 78 after additional therapy, and 98 at extended follow-up of 24 months (Q1-Q3: 21-29 months) after randomization. Echocardiographic GLS was measured in 89 patients at baseline, in 87 at the completion of anthracycline therapy, in 64 after additional therapy, and in 81 at extended follow-up, whereas 120 patients had cTns and NT-proBNP analyzed at baseline, 114 after the first cycle with anthracyclines, 115 at the completion of anthracyclines, and, for those concerned, 81 after additional treatment. Cardiovascular biomarkers were analyzed in 103 patients at extended follow-up. On the basis of these measurements, CTRCD could be assessed in 115 of 120 participants after the first cycle with anthracyclines, in 115 of 120 at the completion of anthracyclines, for those concerned in 81 of 86 after additional treatment, and in 103 of 120 at extended follow-up (Figure 2).
Table 1.
Baseline Characteristics of the Study Population
| All (N = 118) | No CTRCD (n = 58) | CTRCD (n = 60) | |
|---|---|---|---|
| Age at recruitment, y | 49 (43-58) | 46 (43-53) | 53.5 (45.5-63)a |
| Height, cm | 168 (163-171) | 168 (163-171) | 168 (163-170) |
| Weight, kg | 71 (63-79) | 71 (63-78) | 72.5 (63-81) |
| Body mass index, kg/m2 | 25.0 (22.8-28.0) | 24.4 (22.8-27.0) | 25.4 (22.4-28.6) |
| Systolic blood pressure, mm Hg | 130 (120-140) | 125 (116-135) | 134 (121-144)a |
| Diastolic blood pressure, mm Hg | 80 (75-85) | 80 (70-85) | 80 (75-90) |
| Heart rate, beats/min | 66 (61-73) | 64.5 (58-71) | 68 (63-74.5)a |
| Current smoking | 21 (17.8) | 7 (12.1) | 14 (23.3) |
| Hypertension | 8 (6.8) | 2 (3.5) | 6 (10.0) |
| Diabetes | 2 (1.7) | 2 (3.5) | 0 (0.0) |
| Serum creatinine, mg/dL | 0.74 (0.69-0.81) | 0.72 (0.68-0.80) | 0.75 (0.70-0.81) |
| Blood hemoglobin, g/dL | 13.3 (12.7-13.8) | 13.0 (12.6-13.7) | 13.5 (12.9-13.9)a |
| Epirubicin dose, mg/m2 | 240 (240-360) | 240 (240-360) | 360 (240-400)a |
| Trastuzumab | 27 (22.9) | 9 (15.5) | 18 (30.0) |
| Radiotherapy | 75 (63.6) | 39 (67.2) | 36 (60.0) |
| Left-sided radiation | 27 (22.9) | 16 (27.6) | 11 (18.3) |
| Taxanes | 95 (80.5) | 49 (84.5) | 46 (76.7) |
| Study medication | |||
| Candesartan | 60 (50.8) | 31 (53.4) | 29 (48.3) |
| Metoprolol | 57 (48.3) | 31 (53.4) | 26 (43.3) |
| Follow-up, wk | 101 (82-122) | 99 (72-109) | 105 (89-128)a |
| Circulating biomarkers | |||
| cTnT, ng/L | 3 (3-5) | 3 (3-3) | 3 (3-5)a |
| cTnI, ng/L | 0.8 (0.8-1.4) | 0.8 (0.8-1.2) | 1.2 (0.8-1.8)a |
| NT-proBNP, pg/mL | 5.8 (3.8-9.0) | 6.0 (3.8-8.7) | 5.7 (3.5-9.0) |
| Imaging | |||
| LVEF | 63 (60-66) | 63 (61-65) | 63 (59-66) |
| (n = 88) | (n = 47) | (n = 41) | |
| GLS | 21.8 (20.5-23.0) | 22.4 (20.5-23.1) | 21.1 (20.2-22.7) |
Values are median (Q1-Q3) or n (%). Cumulative incidence of CTRCD using the sex-specific cTnT 99th percentile as the upper reference limit. This table includes the 118 of 120 patients who had at least one valid assessment of CTRCD after initiation of anthracyclines.
cTn = cardiac troponin; CTRCD = cancer therapy–related cardiac dysfunction; GLS = global longitudinal strain; LVEF = left ventricular ejection fraction; NT-proBNP = amino-terminal pro–B-type natriuretic peptide.
P < 0.05.
During the observation period, a total of 60 patients (50.8%) fulfilled the new diagnostic criteria for CTRCD when a cTnT value greater than the sex-specific 99th percentile was used as the criterion (Table 2). In the majority of cases, CTRCD was mild and asymptomatic (n = 58 of 118 [49.2%]), peaking at the end of anthracycline therapy when mild asymptomatic CTRCD was diagnosed (n = 55 of 115 [47.8%]). CTRCD was defined by a decline in GLS in 2 of 115 patients (1.7%), by elevated cTnT in 52 patients (45.2%), and by combined decline in GLS and elevated cTnT in 1 patient (0.9%). No symptomatic or moderate or severe asymptomatic CTRCD was observed at this time point.
Table 2.
Point Prevalence of Cancer Therapy–Related Cardiac Dysfunction According to the International Cardio-Oncology Society Definition Using the Sex-Specific Cardiac Troponin T 99th Percentile
| After 1 Cycle (n = 115) | After Anthracyclines (n = 115) | After Trastuzumab or Radiation Therapy (n = 81) | Extended Follow-Up (n = 103) | |
|---|---|---|---|---|
| Symptomatic | ||||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) |
| Asymptomatic | ||||
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Mild | 1 (0.9) | 55 (47.8) | 11 (13.6) | 10 (9.7) |
| Total | 1 (0.9) | 55 (47.8) | 12 (14.8) | 11 (10.7) |
Values are n (%).
At the end of adjuvant therapy with trastuzumab and/or radiotherapy, moderate asymptomatic CTRCD, defined by an LVEF decline of ≥10 percentage points to a value between 40% and 49%, was observed in 1 of 81 patients (1.2%), and mild asymptomatic CTRCD was diagnosed in 11 patients (13.6%) by decline in GLS (n = 2 [2.5%]) or cTnT elevation (n = 9 [11.1%]). No symptomatic or severe asymptomatic CTRCD was observed at this time point.
At extended follow-up of 24 months (Q1-Q3: 21-29 months) after randomization, 1 of 103 patients (1.0%) experienced moderate symptomatic CTRCD with an LVEF decline of ≥10 percentage points to a value between 40% and 49%, requiring outpatient HF therapy. Ten patients (9.7%) were diagnosed with mild asymptomatic CTRCD by a decline in GLS (n = 3 [2.9%]) or cTnT elevation (n = 7 [6.8%]). There was no moderate or severe asymptomatic CTRCD at extended follow-up. Of 15 participants without assessment at extended follow-up, only 3 had mild CTRCD as assessed by sex-specific cTnT URL at the end of anthracyclines.
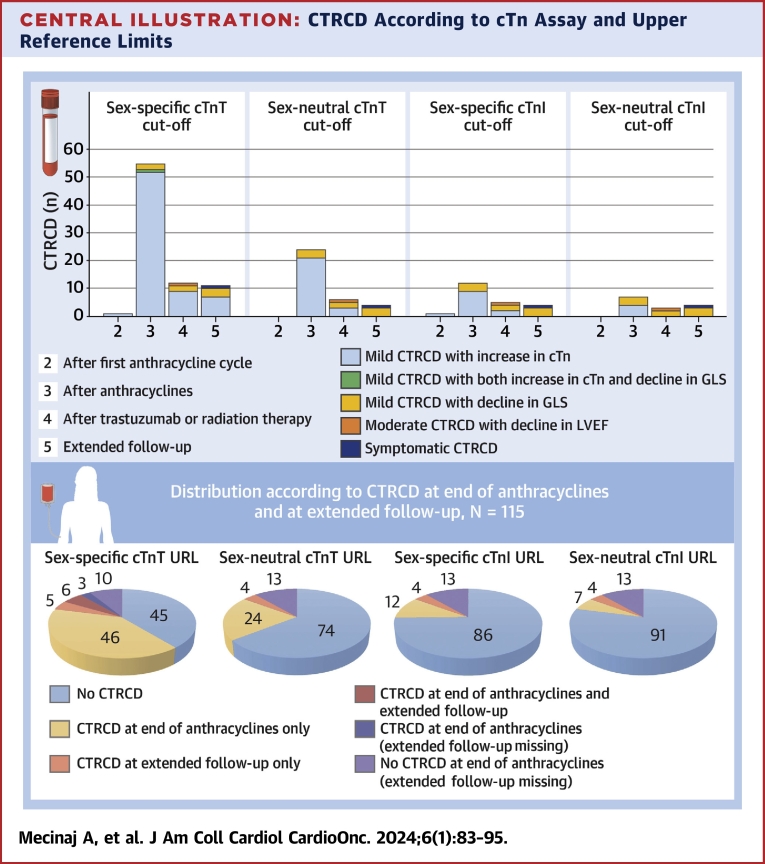
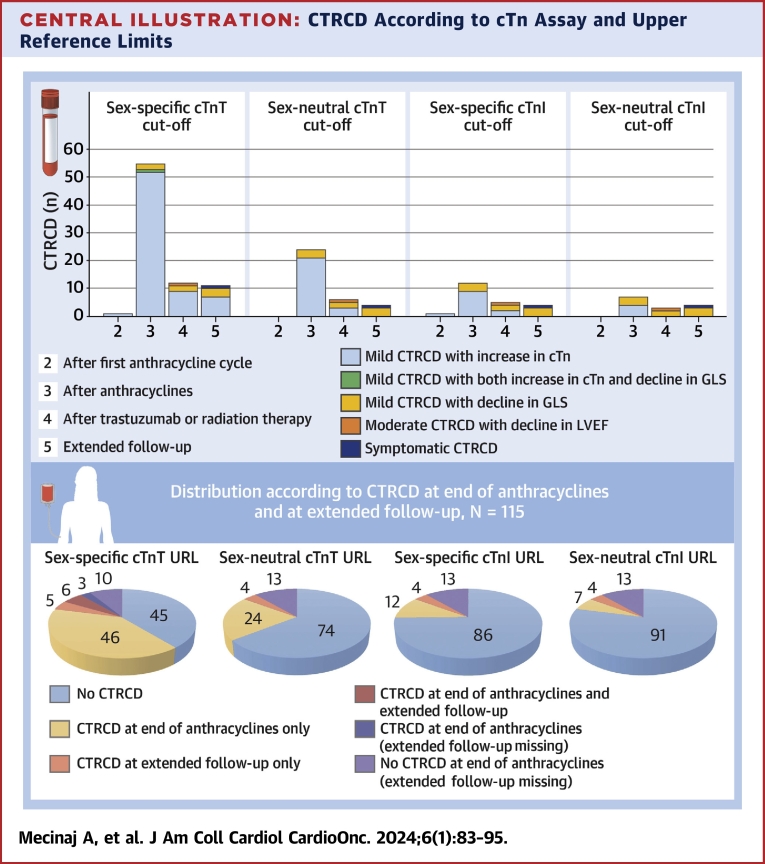
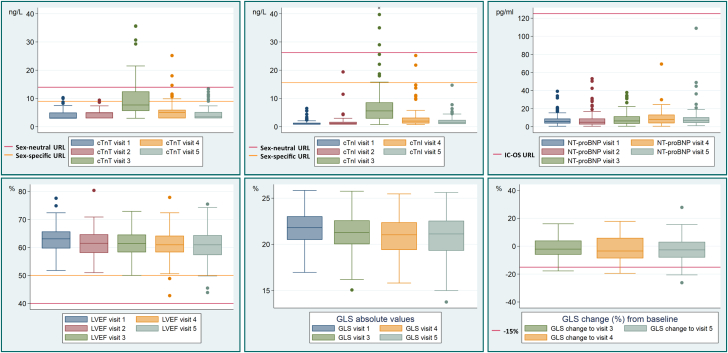
The point prevalence of CTRCD was lower at all time points when defined by cTnI with a cutoff above the sex-specific 99th percentile URL. At the end of anthracycline therapy, the point prevalence of mild asymptomatic CTRCD was 10.4%, after radiotherapy and/or trastuzumab 4.9%, and at extended follow-up 2.9%. When sex-neutral cTnT and cTnI 99th percentile URLs were used to define CTRCD, the point prevalence was lower for both assays: after anthracycline therapy, the point prevalence of mild asymptomatic CTRCD was 20.9% and 6.1%, after radiotherapy and/or trastuzumab 6.2% and 2.5%, and at extended follow-up 2.9% and 2.9%, respectively (Central Illustration, Table 3). At no time point were NT-proBNP values ≥125 ng/L measured (Figure 3, Supplemental Table 1); as a result, the focus of the study was on cTn.
Central Illustration.
CTRCD According to cTn Assay and Upper Reference Limits
(Top) Distribution of cancer therapy–related cardiac dysfunction (CTRCD) by cardiac troponin (cTn) definition. (Bottom) Distribution of CTRCD by cTn definition at the end of anthracycline therapy and at extended follow-up in the 115 patients with assessment at the end of anthracycline therapy. GLS = global longitudinal strain; LVEF = left ventricular ejection fraction; URL = upper reference limit.
Table 3.
Distribution of CTRCD Diagnosis at Different Time Points According to Different Troponin Upper Reference Limit
| Baselinea (n = 120) | After 1 Cycle (n = 115) | After Anthracyclines (n = 115) | After Trastuzumab or Radiation Therapy (n = 81) | Extended Follow-Up (n = 103) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex-specific cTnI | |||||||||||
| No CTRCD | CTRCD | No CTRCD | CTRCD | No CTRCD | CTRCD | No CTRCD | CTRCD | No CTRCD | CTRCD | ||
| Sex-specific cTnT | No CTRCD | 118 (98) | 0 (0) | 113 (98) | 1 (1) | 60 (52) | 0 (0) | 68 (84) | 1 (1) | 92 (89) | 0 (0) |
| CTRCD | 2 (2) | 0 (0) | 1 (1) | 0 (0) | 43 (37) | 12 (10) | 8 (10) | 4 (5) | 7 (7) | 4 (4) | |
| Sex-neutral cTnI | |||||||||||
| No CTRCD | CTRCD | No CTRCD | CTRCD | No CTRCD | CTRCD | No CTRCD | CTRCD | No CTRCD | CTRCD | ||
| Sex-neutral cTnT | No CTRCD | 120 (100) | 0 (0) | 115 (100) | 0 (0) | 90 (78) | 1 (1) | 75 (93) | 0 (0) | 99 (96) | 0 (0) |
| CTRCD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 18 (16) | 6 (5) | 3 (4) | 3 (4) | 0 (0) | 4 (4) | |
Values are n (%). The table is to be interpreted as follows: 118 of 120 patients at baseline were defined as “no CTRCD” by both sex-specific cTnT and cTnI; 2 patients fulfilled the CTRCD criteria defined by sex-specific cTnT but not cTnI.
cTn = cardiac troponin; CTRCD = cancer therapy–related cardiac dysfunction.
At baseline, we report the number and percentage of patients who fulfilled the CTRCD criteria prior to the initiation of adjuvant therapy.
Figure 3.
Biomarker Concentrations and Systolic Function at Each Study Time Point
Distribution of cardiac troponin (cTn) T and I, amino-terminal pro–B-type natriuretic peptide (NT-proBNP), LVEF, GLS, and relative change in GLS from baseline at baseline (visit 1), after 1 anthracycline cycle (visit 2), at the end of anthracyclines (visit 3), after trastuzumab or radiation therapy (visit 4), and at extended follow-up (visit 5). Horizontal lines represent the different cutoff values; x indicates an outlier (82.3 ng/L). Abbreviations as in Figure 1.
Prediction of CTRCD
The patients who at any time point during the observation period developed CTRCD as defined by sex-specific cTnT were older (OR per 10 year increase: 1.68; 95% CI: 1.27-2.22), had higher systolic blood pressure at baseline (OR per 10 mm Hg increase: 1.34; 95% CI: 1.12-1.62), and were scheduled for higher doses of epirubicin (OR per 100 mg/m2 increase: 1.62; 95% CI: 1.15-2.30) compared with patients who did not develop CTRCD. The association between systolic blood pressure and the development of CTRCD was attenuated, whereas treatment with trastuzumab was associated with higher risk for CTRCD when the sex-neutral cTnT URL was used. None of these risk factors was predictive of the development of CTRCD when assessed with cTnI (Table 4).
Table 4.
Univariable Associations Between Risk Factors and the Occurrence of CTRCD During the Observation Period
| Sex-Specific cTnT (n = 60) |
Sex-Specific cTnI (n = 18) |
Sex-Neutral cTnT (n = 31) |
Sex-Neutral cTnI (n = 13) |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (per decade) | 1.68 | 1.27-2.22 | 1.46 | 0.78-2.73 | 1.75 | 1.20-2.56 | 1.34 | 0.65-2.76 |
| Systolic blood pressure (per 10 mm Hg) | 1.34 | 1.12-1.62 | 1.25 | 0.79-1.97 | 1.23 | 0.95-1.59 | 1.18 | 0.69-2.02 |
| Epirubicin dose (per 100 mg/m2) | 1.62 | 1.15-2.30 | 1.71 | 0.75-3.91 | 2.32 | 1.40-3.85 | 1.51 | 0.58-3.95 |
| Trastuzumab | 1.57 | 0.89-2.75 | 2.13 | 0.53-8.47 | 2.33 | 1.13-4.80 | 1.64 | 0.34-7.90 |
Mixed-effects logistic regression analyses of risk for CTRCD during adjuvant therapy according to different cTn upper reference limits in the 118 patients who had at least one valid assessment of CTRCD after initiation of anthracyclines. Separate models for each risk factor.
Abbreviations as in Table 3.
Prognostic value of CTRCD
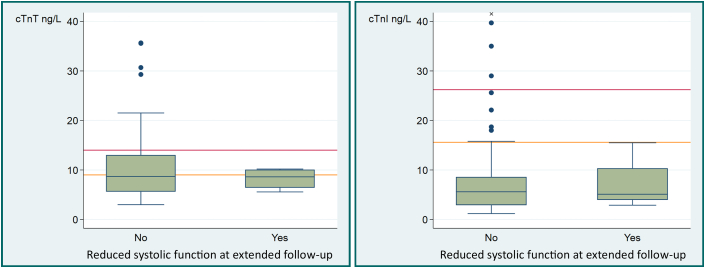
Mild CTRCD was highly prevalent in our group, primarily after completed treatment with anthracyclines. However, the occurrence of CTRCD at the end of anthracycline therapy by any of the biomarker definitions used did not predict CTRCD or decline in systolic function as assessed by reduced GLS or LVEF at extended follow-up of 24 months after randomization. For example, using the sex-specific cTnT cutoff, the ORs were 1.17 (95% CI: 0.33-4.1) and 0.96 (95% CI: 0.13-7.1), respectively (Table 5). Importantly, none of the 4 patients with reduced systolic function at extended follow-up had troponin elevations greater than the sex-specific cTnI URL or the sex-neutral cTnT or cTnI URLs at the end of anthracyclines (Figure 4). Furthermore, there was no correlation between change in cTnT or cTnI from baseline to the end of anthracycline therapy and change in systolic function from baseline to extended follow-up (Supplemental Table 2).
Table 5.
CTRCD at the End of Anthracycline Therapy and at Extended Follow-Up
| CTRCD at the End of Anthracyclines (n = 115) |
CTRCD at Extended Follow-Up (n = 103) |
|
|---|---|---|
| No | Yes | |
| Sex-specific cTnT | ||
| No | 45 (44) | 5 (5) |
| Yes | 46 (45) | 6 (6) |
| Sex-specific cTnI | ||
| No | 86 (84) | 4 (4) |
| Yes | 12 (12) | 0 (0) |
| Sex-neutral cTnT | ||
| No | 74 (73) | 4 (4) |
| Yes | 24 (24) | 0 (0) |
| Sex-neutral cTnI | ||
| No | 91 (89) | 4 (4) |
| Yes | 7 (7) | 0 (0) |
Values are n (%). The table is to be interpreted as follows: 115 and 103 participants were assessed for CTRCD at the end of anthracycline therapy and at extended follow-up, respectively, and 102 participants were assessed at both time points. When assessed using the sex-specific cTnT upper reference limit as a cutoff, 44% did not have CTRCD at either time point, 45% had CTRCD the end of anthracycline therapy but not at extended follow-up, 5% had CTRCD at extended follow-up only, and 6% had CTRCD at both time points.
Abbreviations as in Table 3.
Figure 4.
Troponin Levels After Anthracyclines by Systolic Function at Extended Follow-Up
Distribution of cardiac troponin (cTn) T and I according to whether systolic function was reduced at extended follow-up. Reduced systolic function was defined as a left ventricular ejection decline of ≥10 percentage points to a value between 40% and 49% or a decline in global longitudinal strain of ≥15% from baseline. Horizontal orange and red lines represent sex-specific and sex-neutral upper reference limits, and x indicates an outlier (82.3 ng/L).
Treatment efficacy
In a post hoc analysis, the effect of intervention with metoprolol vs placebo and candesartan vs placebo on the incidence of CTRCD was examined. There was no association between randomization to metoprolol vs placebo or to candesartan vs placebo and the CTRCD point prevalence at any time point or cumulative incidence by any of the definitions (Table 6).
Table 6.
Risk for CTRCD According to study Medication Randomization
| Sex-Specific cTnT |
Sex-Specific cTnI |
Sex-Neutral cTnT |
Sex-Neutral cTnI |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| CTRCD at the end of anthracycline therapy | ||||||||
| Metoprolol vs placebo | 0.63 | 0.30-1.32 | 0.51 | 0.14-1.80 | 0.73 | 0.29-1.81 | 0.81 | 0.17-3.78 |
| Candesartan vs placebo | 1.04 | 0.50-2.17 | 0.91 | 0.27-3.00 | 1.11 | 0.45-2.73 | 0.34 | 0.06-1.86 |
| CTRCD at extended follow-up | ||||||||
| Metoprolol vs placebo | 1.31 | 0.37-4.59 | 0.34 | 0.03-3.38 | 0.34 | 0.03-3.38 | 0.34 | 0.03-3.38 |
| Candesartan vs placebo | 0.30 | 0.08-1.21 | 0.29 | 0.03-2.87 | 0.29 | 0.03-2.87 | 0.29 | 0.03-2.87 |
| CTRCD at any time point during follow-up | ||||||||
| Metoprolol vs placebo | 0.67 | 0.40-1.12 | 0.34 | 0.10-1.21 | 0.55 | 0.26-1.13 | 0.41 | 0.10-1.75 |
| Candesartan vs placebo | 0.91 | 0.55-1.51 | 0.63 | 0.18-2.17 | 0.95 | 0.47-1.91 | 0.44 | 0.10-2.01 |
Risk for CTRCD according to study medication and different cTn upper reference limits. Univariable logistic regression analyses of risk of CTRCD at different time points during the observation period and separate mixed-effects logistic regression analyses of the risk for CTRCD at any time point during follow-up.
Abbreviations as in Table 3.
Discussion
In this analysis of the PRADA trial, we evaluated the new definition of CTRDC during adjuvant therapy for early breast cancer. We demonstrate 5 key findings: 1) in patients with early breast cancer receiving adjuvant anthracycline-based chemotherapy, the cumulative incidence of mild CTRCD according to the IC-OS definition is high, with the highest point prevalence at the end of anthracycline therapy; 2) the CTRCD diagnosis was driven primarily by a high incidence of cTn concentrations greater than the 99th percentile URL; 3) there was a marked difference in the incidence of CTRCD assessed by cTnI vs cTnT and between sex-specific and sex-neutral 99th percentile URLs for cTn; 4) occurrence of CTRCD at the end of anthracycline therapy was not associated with subsequent CTRCD by any of the biomarker definitions used; and 5) in a post hoc analysis, neither metoprolol nor candesartan was significantly associated with reduced incidence of CTRCD during anthracycline therapy or at extended follow-up of 24 months after randomization.
Impact of different biomarker criteria on the incidence estimates of CTRCD
In the present study, the reported incidence of CTRCD differed markedly according to the diagnostic criteria used. In earlier clinical trials, cardiotoxicity was defined as an absolute decline in LVEF (often 10%) to a level less than a cutoff value of LVEF (often 50% or 53%) or clinical symptoms of HF. The lack of a uniform definition has impeded direct comparison among trials. Increasing sensitivity of cardiac imaging indexes such as GLS and cardiac-specific biomarkers such as cTn measured with high-sensitivity assays has resulted in enhanced ability to detect myocardial injury. Hence, it has become more common to include these variables in the CTRCD definition.9,10 Although the variability in incidence in previous studies may partially be explained by differences in cardiovascular risk profiles at baseline and anthracycline dose, the sensitivity and specificity of different diagnostic criteria may also differ. The recently published IC-OS consensus definition of cardiotoxicity and the ESC cardio-oncology guidelines will make it easier to determine CTRCD incidence across studies and to better define the best preventive strategies and management of CTRCD.3,4 However, the guidelines do not differentiate among the biomarker assays and do not clarify whether sex-specific or sex-neutral definitions should be applied. In our study, the incidence of mild cardiotoxicity varied substantially according to whether cTnI or cTnT measurements and sex-specific vs -neutral cutoff values were used. By defining CTRCD according to the sex-specific URL for cTnT, the point prevalence of mild CTRCD at the end of anthracycline therapy for early breast cancer was high, even in a cohort with a relatively low CVD risk profile, and much higher than if a sex-neutral URL for cTnI was used. Therefore, our findings demonstrate that the incidence rate of CTRCD according to the new IC-OS definition will strongly depend on choice of biomarker assay and whether sex-specific vs neutral URLs are used.
Why do cTnI vs cTnT measurements result in markedly different incidence estimates of mild CTRCD?
In the recent guidelines, cTnI and cTnT elevations greater than the 99th percentile are treated interchangeably.4 Most previous studies demonstrating increases in cTns after anthracycline therapy have used either cTnI or cTnT assays, and there are few studies reporting head-to head comparisons between cTnT vs cTnI in response to cancer therapy.11 However, the present results strongly suggest that the use of different cTn assays will markedly affect CTRCD incidence estimates. These observations also raise questions concerning the reason for these discrepancies. Both biological and analytical factors can theoretically play a role. Interesting differences in cTnT and cTnI cardiomyocyte release kinetics, circulating half-life, and analytical characteristics have been described.12 In the setting of acute myocardial injury, cTnT has been shown to have a slower release pattern and longer circulating half-life than cTnI, and the different kinetics may potentially lead to different results according to the timing of blood sampling relative to the peak troponin concentration after chemotherapy. Recently, an international multicenter study assessed the diagnostic accuracy of high-sensitivity cTn in 8,267 patients with chest pain with and without histories of cancer. This study demonstrated significantly lower diagnostic accuracy of cTnT for non–ST-segment elevation myocardial infarction in patients with cancer compared with patients without cancer, whereas the diagnostic accuracy of cTnI was comparable between the 2 groups. This may suggest that cardiac effects of cancer and cardiotoxic cancer therapies are associated with a more pronounced cTnT than cTnI release.13 Both cTnT and cTnI are strongly associated with cardiac disease and with similar prognostic value for all-cause mortality in the general population. However, whereas cTnI is more predictive of cardiovascular mortality, cTnT has been more strongly associated with noncardiovascular mortality, suggesting a potential increased specificity for cardiac injury for cTnI over cTnT.14 In 2 recent studies of patients with skeletal muscle disorders, cTnT was more commonly elevated than cTnI,15,16 and this was generally not attributable to cardiac disease.15 Possible explanations are skeletal–cardiac muscle troponin T cross-reactivity and cTnT expression in skeletal muscle, and in our cohort, concurrent anthracycline skeletal muscle toxicity may have contributed to the higher prevalence of cTnT greater than the URL compared with cTnI.
However, analytical factors, in particular the definition of the 99th percentile URLs for different cTn assays, are likely to play a more important role. The 99th percentile URLs are currently established by manufacturers on the basis of a healthy reference population. Unfortunately, there is no consensus on how to define healthy. Moreover, both sex and age of the healthy reference population may affect the 99th percentile URL, and in particular, the age distributions of reference populations may differ among manufacturers. Further complicating this is that the choice of using the 99th percentile rather than, for instance, the 97.5th percentile17 makes the URL susceptible to the influence of outliers, and this may especially true for sex-specific URLs because of the limited number of subjects in the healthy reference population with values greater than the 99th percentile. Accordingly, differences in the healthy cohorts used by different manufacturers to define the 99th percentile URLs may have led to substantial differences in the 99th percentile URLs for different cTn assays. As a consequence, 99th percentile URLs for cTnT and cTnI may not be bioequivalent,18 leading to major inconsistencies in the diagnosis of CTRCD.
Should sex-specific vs -neutral 99th percentile be used to define CTRCD?
The International Federation of Clinical Chemistry and Laboratory Medicine committee for cardiovascular biomarkers recommends the use of specific URLs for cTn,19 although the clinical consequences of using sex-specific vs -neutral URLs in the setting of acute coronary syndrome has been debated.20 However, it is clear from the present data that the choice of sex-specific vs -neutral cTnT and cTnI URLs for the diagnosis of CTRCD will have a substantial impact on the reported incidence rates. To avoid heterogeneity of reporting and unnecessary confusion among patients and clinicians, it would be helpful if a revised version of the IC-OS definition specified whether sex-specific vs sex-neutral URLs should be used.
Prognostic value of CTRCD diagnosed by cardiac biomarkers
Data on whether an early rise in troponins after anthracycline therapy can predict later cardiotoxicity have been conflicting. For instance, in a prospective, observational study of 323 patients with breast cancer treated with anthracyclines and/or trastuzumab, a cTnT elevation >14 ng/L at the end of anthracycline therapy was associated with a twofold increase in risk for CTRCD, defined as a ≥10% decline in LVEF to a value <50% during a follow-up period of 3.7 years.21 However, similar to our study, cTnT elevations were common, and changes over time in cTnT were not associated with CTRCD. Furthermore, in the recent Cardiac CARE trial, a multicenter, prospective, randomized, open-label, blinded endpoint trial of 175 patients with breast cancer or non-Hodgkin lymphoma randomized to standard care alone vs standard care plus cardioprotection with candesartan and carvedilol depending on cTnI concentrations during anthracycline therapy, no association between cTnI concentrations and change in LVEF was observed.22
A meta-analysis published in 2020 included data from 61 trials and 5,691 patients with cancer, 43% of whom were treated for breast cancer. In that study, elevated cTn values were common after anticancer therapy (22.4%), and the rate of cardiotoxicity as indicated by LVEF decrease was 17.0%. However, the definitions of cTn elevation were not uniform among studies, and the majority of studies did not use high-sensitivity assays. Patients with elevated cTn had higher odds of left ventricular dysfunction, with an OR of 11.9, but the investigators reported substantial heterogeneity and significant reporting bias.11 In our study, the median anthracycline dose was relatively low (median doxorubicin equivalent dose 161 mg/m2; range: 161-268 mg/m2), but even so, mild asymptomatic CTRCD at the end of anthracycline therapy was highly prevalent. However, at extended follow-up, there was only 1 case of symptomatic CTRCD and, depending on definition, 3 to 10 mild asymptomatic cases (Table 3). Notably, CTRCD at the end of anthracycline therapy was not predictive of CTRCD as defined by the IC-OS criteria or of reduced systolic function at extended follow-up. Until there is more evidence on the optimal cutoff levels and the clinical implications of mild asymptomatic CTRCD, we recommend caution when interpreting isolated troponin elevations during adjuvant therapy.
Intervention
Previous studies have shown that neurohormonal blockade may attenuate reduction in LVEF during treatment of early breast cancer with anthracyclines with or without trastuzumab. In a systematic review and meta-analysis of beta-blockers and renin-angiotensin system inhibitors for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer, there was only a 2% absolute difference in LVEF between the intervention and placebo groups.23 In the primary results of the PRADA trial, candesartan attenuated the decline in LVEF during adjuvant therapy for early breast cancer,5 and in both the PRADA6 and CECCY (Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity)24 trials, beta-blockade attenuated the increase of cTns during anthracycline therapy, but long-term follow-up showed no difference in change in LVEF in either trial.7,25 In the present study, neither metoprolol nor candesartan attenuated the incidence of CTRCD as defined by the new IC-OS definition. This observation is clinically relevant, as the 2022 ESC guidelines recommend considering treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and/or beta-blockers in those with mild asymptomatic cardiotoxicity. Initiating cardioprotection in patients who are unlikely to benefit should be undertaken with caution, as cardioprotective therapy with neurohormonal antagonists may cause side effects such as hypotension, asthenia, electrolyte imbalance, and worsening of renal function.22
Study limitations
Patients included in a single-center, randomized controlled trial in a Scandinavian country may not be generalizable to patients with breast cancer from other countries and of other ethnicities. However, even in this cohort with a relatively low prevalence of pre-existing cardiovascular disease and risk factors, the cumulative incidence of mild asymptomatic CTRCD was high, and we believe that in a higher risk cohort, the CTRCD rates would likely have been even higher and the differences according to biomarker assays and 99th percentile URL definitions even more pronounced.
Not all patients had all measurements at all time points. By design, echocardiography was not performed after the first cycle of anthracyclines, and only patients who received trastuzumab and/or radiotherapy had visits.4 Because of challenges in achieving sufficient echocardiographic image quality after breast cancer surgery, there are fewer GLS measurements than CMR studies and biomarker assessments at each time point. However, in the PRADA trial, the primary endpoint was change in LVEF assessed on CMR, and we do not believe the missing data to be due to differential dropout. The great majority of participants without extended follow-up examinations did not have CTRCD at the end of anthracycline therapy. We therefore believe it to be unlikely that a complete data set would change the finding that CTRCD at the end of anthracyclines did not predict CTRCD at extended follow-up. The median follow-up time of 24 months may be too short to assess the predictive value of CTRCD on long-term anthracycline toxicity.
Conclusions
The incidence of mild, asymptomatic CTRCD according to the recent IC-OS definition was high during adjuvant therapy for early breast cancer and driven mainly by cTn elevation. The choice of cTn assay and the use of sex-specific vs -neutral decision limits have substantial impact on CTRCD incidence. CTRCD was not prevented by neurohormonal blockade, nor did CTRCD during anthracycline therapy predict subsequent CTRCD. These observations raise questions concerning the interpretation and clinical implications of the new definition of mild asymptomatic CTRCD.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The recent ESC cardio-oncology guidelines include troponin elevations greater than the 99th percentile in the definition of asymptomatic CTRCD but do not distinguish between cTnI and cTnT or specify whether sex-specific vs -neutral URLs should be used. In the present study, the cumulative incidence of mild, asymptomatic CTRCD was high and differed markedly according to the biomarker criteria applied. Furthermore, mild asymptomatic CTRCD was not prognostic of subsequent CTRCD at 24 months postrandomization.
TRANSLATIONAL OUTLOOK: The large discrepancy in the incidence of mild, asymptomatic CTRCD according to different troponin assays and URLs challenges the utility and clinical implications of the new definition. Further research is needed to define the role and best cutoff values for cTnT and cTnI to diagnose asymptomatic CTRCD.
Funding Support and Author Disclosures
This work was supported by the South-Eastern Norway Regional Health Authority, the University of Oslo, the Extra Foundation for Health and Rehabilitation, Norway, the Norwegian Cancer Society, and Akershus University Hospital. Study medications and matching placebos were provided free of charge by AstraZeneca. Reagents for the analysis of high-sensitivity cTnI were provided by Abbott Diagnostics. Dr Gulati has received speaker honoraria from Novartis, AstraZeneca, and Bristol Myers Squibb. Dr Omland has served on advisory boards for Abbott Diagnostics, Roche Diagnostics, and Bayer; has received research support from Abbott Diagnostics, Novartis, and Roche Diagnostics via Akershus University Hospital; and has received speaker or consulting honoraria from Roche Diagnostics, Siemens Healthineers, and CardiNor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the Data and Safety Monitoring Board; the staff of the Clinical Research Unit, Division of Medicine; and the radiographers at the Cardiac Magnetic Resonance Unit of the Department of Diagnostic Imaging, Akershus University Hospital, for assistance with all aspects of trial execution. The authors acknowledge the generous support from Stiftelsen Kristian Gerhard Jebsen (K.G. Jebsen Center for Cardiac Biomarkers, grant SKGJ-MED-024) to Drs Mecinaj, Gulati, Røsjø, Omland, and Heck.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and references, please see the online version of this paper.
Appendix
References
- 1.Strongman H., Gadd S., Matthews A., et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gernaat S.A.M., Ho P.J., Rijnberg N., et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164:537–555. doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyon A.R., Lopez-Fernandez T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 5.Gulati G., Heck S.L., Ree A.H., et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulati G., Heck S.L., Rosjo H., et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heck S.L., Mecinaj A., Ree A.H., et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): extended follow-up of a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. 2021;143:2431–2440. doi: 10.1161/CIRCULATIONAHA.121.054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collinson P.O., Saenger A.K., Apple F.S., Ifcc C.C. High sensitivity, contemporary and point-of-care cardiac troponin assays: educational aids developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin Chem Lab Med. 2019;57:623–632. doi: 10.1515/cclm-2018-1211. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Sendon J., Alvarez-Ortega C., Zamora Aunon P., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41:1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 10.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N., et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4:1007–1018. doi: 10.1001/jamacardio.2019.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michel L., Mincu R.I., Mahabadi A.A., et al. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22:350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 12.Starnberg K., Friden V., Muslimovic A., et al. A possible mechanism behind faster clearance and higher peak concentrations of cardiac troponin I compared with troponin T in acute myocardial infarction. Clin Chem. 2020;66:333–341. doi: 10.1093/clinchem/hvz003. [DOI] [PubMed] [Google Scholar]
- 13.Bima P., Lopez-Ayala P., Koechlin L., et al. Chest pain in cancer patients. J Am Coll Cardiol CardioOnc. 2023;5:591–609. doi: 10.1016/j.jaccao.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh P., Preiss D., Hayward C., et al. Cardiac troponin T and troponin I in the general population. Circulation. 2019;139:2754–2764. doi: 10.1161/CIRCULATIONAHA.118.038529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.du Fay de Lavallaz J., Prepoudis A., Wendebourg M.J., et al. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation. 2022;145:1764–1779. doi: 10.1161/CIRCULATIONAHA.121.058489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid J., Liesinger L., Birner-Gruenberger R., et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. 2018;71:1540–1549. doi: 10.1016/j.jacc.2018.01.070. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 18.Wildi K., Gimenez M.R., Twerenbold R., et al. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation. 2015;131:2032–2040. doi: 10.1161/CIRCULATIONAHA.114.014129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aakre K.M., Saenger A.K., Body R., et al. Analytical considerations in deriving 99th percentile upper reference limits for high-sensitivity cardiac troponin assays: educational recommendations from the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin Chem. 2022;68:1022–1030. doi: 10.1093/clinchem/hvac092. [DOI] [PubMed] [Google Scholar]
- 20.Twerenbold R., Boeddinghaus J., Nestelberger T., et al. How to best use high-sensitivity cardiac troponin in patients with suspected myocardial infarction. Clin Biochem. 2018;53:143–155. doi: 10.1016/j.clinbiochem.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Demissei B.G., Hubbard R.A., Zhang L., et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriksen P.A., Hall P., MacPherson I.R., et al. Multicenter, prospective, randomized controlled trial of high-sensitivity cardiac troponin I–guided combination angiotensin receptor blockade and beta-blocker therapy to prevent anthracycline cardiotoxicity: the Cardiac CARE trial. Circulation. 2023;148(21):1680–1690. doi: 10.1161/CIRCULATIONAHA.123.064274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewinter C., Nielsen T., Edfors L., et al. A systematic review and meta-analysis of beta-blockers and inhibitors of the renin-angiotensin system for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer. Eur Heart J. 2023;43(27):2562–2569. doi: 10.1093/eurheartj/ehab843. [DOI] [PubMed] [Google Scholar]
- 24.Avila M.S., Ayub-Ferreira S.M., de Barros Wanderley M.R., Jr., et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 25.Ayub-Ferreira S.M., Avila M., Brandao S., et al. Carvedilol for prevention of chemotherapy-induced cardiotoxicity: final results of the prospective, randomized, double-blind, placebo controlled CECCY trial. J Am Coll Cardiol. 2020;75 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.