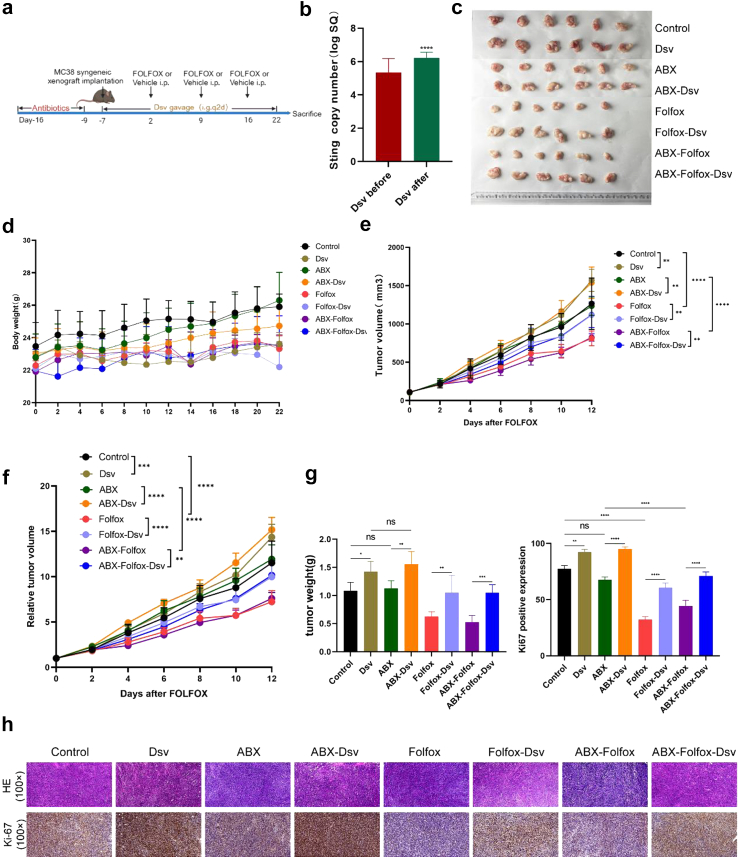
Fig. 3.
Treatment with D. desulfuricans significantly diminished the anticancer efficacy of the FOLFOX regimen. (a) The in vivo experimental design of this study. 1 × 106 MC38 cells were subcutaneously injected into the lateral flank of the mice. D. Desulfuricans were delivered via gavage at a dose of 1 × 109 colony-forming units (CFU) per mouse every other day. FOLFOX chemotherapy was administered according to the dosages of oxaliplatin (6 mg/kg), 5-FU (50 mg/kg), and calcium folinate (90 mg/kg), with oxaliplatin administered 2 h after 5-FU once a week via intraperitoneal injection. (b) QPCR was used to compare the absolute abundance of D. desulfuricans in mouse faecal samples before and 7 days after oral administration (n = 24 mice/group). (c, e, and f) Comparison of tumour volumes among groups (n = 6 mice/group). (d) Changes in body weight of mice during the treatment period (n = 6 mice/group). (g) Comparison of tumour weights among different groups (n = 6 mice/group). (h) Histological images of tumour tissues stained with H&E and IHC staining for Ki-67 expression in various groups (scale bar, 100 μm). The results are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.001. Statistical significance was determined using one-way ANOVA and Tukey's test for multiple comparisons.