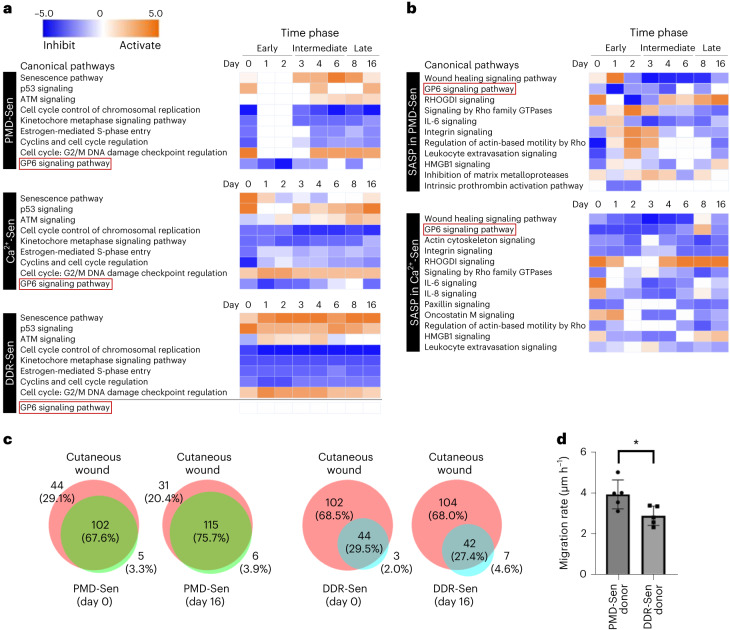
Fig. 7. Transcriptional profiling by RNA-seq reveals potential in vivo functions of PMD-dependent senescent cells.
a, Heat maps generated by IPA comparison analysis show top canonical pathways affected by the differentially expressed mRNAs in PMD-Sen, Ca2+-Sen and DDR-Sen. See also Supplementary Fig. 4. PMD-Sen, PMD-dependent senescent cells; Ca2+-Sen, Ca2+-dependent senescent cells; DDR-Sen, DNA damage response-dependent senescent cells. Orange and blue indicate positive and negative activation z-score, respectively; white indicates no activation. Red outlines highlight the GP6 signaling pathway, which is inhibited in PMD-Sen and Ca2+-Sen but not in DDR-Sen. b, Heat maps generated by IPA comparison analysis show top canonical pathways affected by the SASP factors differentially expressed in PMD-Sen and Ca2+-Sen. See also Supplementary Fig. 5. c, Overlapping canonical pathways in PMD-Sen and cutaneous wounds and in DDR-Sen and cutaneous wounds. RNA-seq results of cutaneous wounds were obtained from the public datasets (GSE141814). See also Extended Data Fig. 9. Day 0 and day 16 are shown. d, PMD-Sen cells accelerate wound healing in vitro in a paracrine manner more than DDR-Sen cells do. Young cells (recipient) were co-cultured with PMD-Sen cells (donor) or DDR-Sen cells (donor). Cell-free gaps (500 μm) were made in the layer of young cells (recipient). Cell migration rate was calculated based on the filled cell-free area in 36 h. See also Extended Data Fig. 10. *P < 0.05, exact value: 0.0254, by two-tailed unpaired Student’s t-test. Data in d are presented as mean (horizontal bars) ± s.d. (whiskers) of five biological replicates.