Abstract
Introduction
The study aimed to evaluate the effectiveness and safety of brivaracetam (BRV) as conversion monotherapy in adults with focal epilepsy treated in the context of real-world clinical practice.
Methods
This was a retrospective, observational, non-interventional study in adults with focal epilepsy who converted to BRV monotherapy following the withdrawal of background antiseizure medications (ASMs). Primary effectiveness outcome was the retention rate of BRV as single ASM at 6 and 12 months. Secondary outcomes included the 6- and 12-month rates of seizure freedom. Safety and tolerability outcomes included the frequency and type of adverse events (AEs) and the occurrence of treatment discontinuation due to AEs.
Results
A total of 44 participants with a median age of 63.5 (interquartile range 44–73.5) years were included; 17 subjects were seizure free at baseline, and 9 of them switched from levetiracetam because of lack of tolerability. The retention rate of BRV monotherapy was 88.6% (39/44) at 6 months and 83.9% (26/31) at 12 months. The rates of seizure freedom were 72.7% (32/44) in subjects with 6-month follow-up and 58.1% (18/31) in subjects with 12-month follow-up. The median maintenance dosage of BRV monotherapy was 150 (100–200) mg/day at 6 months and 125 (100–200) mg/day in subjects with 12-month follow-up. Adverse events were recorded in 6/44 (13.6%) participants and led to BRV discontinuation in 2/44 (4.5%) cases. The reported AEs were somnolence (n = 3), fatigue (n = 2), and irritability (n = 1); no serious AEs were experienced. In 21/44 (47.7%) participants, BRV monotherapy resulted from the direct switch from levetiracetam. The rates of treatment retention and seizure freedom at 6 and 12 months were higher among people who switched from levetiracetam to BRV monotherapy.
Conclusion
Brivaracetam may be a valuable treatment of focal seizures in people who converted to monotherapy in a real-life setting.
Keywords: Antiseizure medication, Brivaracetam, Focal seizures, Epilepsy
Key Summary Points
| Conversion to brivaracetam monotherapy in clinical practice was associated with high 6- and 12-month retention. |
| The rates of seizure freedom were 72.7% and 58.1% at 6- and 12-month follow-up. |
| Treatment retention and seizure freedom rates were higher among people who switched from levetiracetam. |
| Brivaracetam monotherapy was generally well tolerated, and somnolence, fatigue, and irritability were the most common adverse events. |
Introduction
Brivaracetam (BRV) is the newest antiseizure medication (ASM) in the racetam class of compounds and was rationally designed to selectively target the synaptic vesicle 2A protein in the brain with high binding affinity. The drug has a favourable pharmacokinetic profile, with a fast entry into the brain by crossing the blood–brain barrier via passive diffusion, a minimal binding to plasma proteins, and a low potential for drug–drug interactions [1–3]. Furthermore, the availability of different formulations allows flexibility in administration.
In 2016, BRV was first approved as adjunctive treatment for people from 16 years of age with partial onset seizures with or without secondary generalization by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), with later approvals to treat also paediatric patients 1 month of age and older (FDA) and 2 years of age and older (EMA). In 2017, BRV was granted a monotherapy license for the treatment of focal-onset seizures by the FDA on the basis of analysis of data from adjunctive therapy trials. This regulatory policy that accepts the extrapolation of the efficacy and safety of drugs approved as adjunctive therapy to their use as monotherapy can improve access to monotherapies with new compounds and increase the armamentarium for the treatment of focal seizures. Differences exist across regulatory bodies, and non-inferiority trials in newly diagnosed epilepsy are still required by the EMA, although the acceptance of add-on studies in support of a monotherapy claim could be considered on a case-by-case basis in the future [4]. Clinical implications might be relevant as regimen based on one single drug may offer potential advantages compared to polypharmacy, including decreased risks of adverse events and pharmacological interactions and improved tolerability and adherence to treatment [5]. Given the restrictions and discrepancies among regulatory agencies, observational and open-label trials can provide preliminary yet useful information about ASMs given as monotherapy.
The aim of this study was to evaluate the effectiveness and safety of BRV as conversion monotherapy in adults with focal epilepsy treated in the context of real-world clinical practice.
Methods
Participants
This was a retrospective, observational, non-interventional study in people with focal epilepsy conducted at 10 epilepsy centres in Italy. Adult (age at least 18 years) subjects attending participating centres who had a diagnosis of epilepsy with focal-onset seizures [6] and converted to BRV monotherapy following the withdrawal of background ASMs between January 2019 and May 2023 were retrospectively identified. Subjects were required to have received background ASMs for at least 2 weeks before starting BRV monotherapy. Only subjects who had a clinical follow-up of at least 6 months from the initiation of BRV as the only ASM were included in the current analysis. The last documented follow-up visit had to take place before the initiation of the chart review; hence, data collection did not influence treatment decisions that were made independently by the treating physicians, according to their own routine clinical practice. Exclusion criteria were history of generalized seizures, alcoholism, drug abuse, conversion disorders or other non-epileptic ictal events, incomplete or unreliable clinical records according to the treating physician.
Data on demographics, clinical history, type of seizures and epilepsy [6], aetiology, previous and concomitant ASMs, seizure frequency at baseline (monthly seizure frequency during the 6 months before the start of BRV monotherapy), and seizure occurrence, adverse events (AEs) and drug withdrawal at 6 (± 1) and 12 (± 1) months after the start of BRV monotherapy were retrieved from clinical records and patient seizures diaries.
The primary effectiveness outcome was the retention rate of BRV as single ASM at 6 and 12 months after the start of BRV monotherapy. Secondary outcomes included the 6- and 12-month rates of seizure freedom under BRV monotherapy, defined as no seizures since at least the previous time point. Sustained seizure freedom, defined as no seizures from the start of BRV monotherapy throughout the 12-month follow-up [7, 8], was also considered. Subjects who discontinued BRV monotherapy were considered to have no seizure freedom at the time of discontinuation and onwards. Safety and tolerability outcomes included the frequency and type of AEs and the occurrence of treatment discontinuation due to AEs recorded from the initiation of BRV monotherapy until the last follow-up.
Statistical Analysis
Data were analysed using descriptive statistics. Values were presented as mean ± standard deviation or median [interquartile range] for continuous variables and as the number (percent) of subjects for categorical variables. Subgroup analyses were performed according to the switch from levetiracetam (LEV) to BRV monotherapy. Comparisons were made using the chi-squared test. Data analysis was performed using STATA/IC 13.1 (StataCorp LLC, College Station, TX, USA). The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [9].
Ethical Approval
The study was approved by the ethical committee of the Marche Polytechnic University, Ancona, Italy (ID CERM 176) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient or the legal representative for the collection and analysis of data and the dissemination of the results.
Results
A total of 44 participants were included in the study. They had a median epilepsy duration of 7.5 [1.5–18.5] years, and 18 (40.9%) were male. The median number of lifetime ASMs received before starting BRV monotherapy was 2 [1–3], and 33 (75.0%) subjects had a history of LEV use. The median age of the participants at the start of BRV monotherapy was 63.5 [44–73.5] years and 17 (38.6%) subjects had been free from seizures during the previous 6 months; the baseline seizure frequency in non-seizure-free subjects was 0.5 [0.3–0.8] seizure per month. The reason to introduce BRV was the lack of efficacy with other ASMs in 16 (36.4%), the occurrence of adverse events with other ASMs in 21 (47.7%), and both reasons in 7 (15.9%) subjects. Baseline characteristics of participants are summarized in Table 1.
Table 1.
Baseline characteristics of participants
| Characteristics | Participants (n = 44) |
|---|---|
| Male sex | 18 (40.9) |
| Age, years | 63.5 [44–73.5] |
| Duration of epilepsy, years | 7.5 [1.5–18.5] |
| Type of seizure | |
| Focal onset | 26 (59.1) |
| Focal to bilateral tonic–clonic | 13 (29.6) |
| Focal onset and focal to bilateral tonic–clonica | 5 (11.3) |
| Aetiology | |
| Structural | 26 (59.1) |
| Metabolic | 1 (2.3) |
| Unknown | 17 (38.6) |
| Any psychiatric comorbidity | 13 (29.5) |
| Number of prior ASMsb | 2 [1–3] |
| Levetiracetam status | |
| Never used | 11 (25.0) |
| Prior use | 33 (75.0) |
| Reason to introduce brivaracetam | |
| Lack of efficacy with other ASMs | 16 (36.4) |
| Adverse events with other ASMs | 21 (47.7) |
| Both | 7 (15.9) |
| Seizure freedom at baseline | 17 (39.0) |
| Baseline monthly seizure frequencyc | 0.5 [0.3–0.8] |
Data are median [IQR] for continuous variables and n (%) for categorical variables
ASM antiseizure medication, BRV brivaracetam, IQR interquartile range
aSubjects presenting both focal onset and focal to bilateral tonic–clonic seizures
bNumber of lifetime ASMs before starting treatment with BRV monotherapy
cBased on the number of seizures during the 6 months before starting BRV monotherapy in non-seizure-free subjects
In 21/44 (47.7%) participants, BRV monotherapy resulted from the direct switch from LEV. People who switched from LEV to BRV had fewer prior ASMs than people who converted to BRV after the discontinuation of other ASMs. Poor tolerability of LEV was the most common reason to introduce BRV in the subgroup of participants who switched from LEV to BRV monotherapy, and poor efficacy of other ASMs was the most common reason to introduce BRV in the subgroup of participants who converted to BRV monotherapy following the withdrawal of other ASMs. Baseline characteristics of participants according to LEV switch are shown in Table 2. In participants who did not switch from LEV (n = 23), BRV was added to a median of 1 [1–1] concomitant ASM and the last ASM before the conversion to BRV monotherapy was lacosamide (n = 6), benzodiazepines (clobazam, clonazepam) (n = 5), carbamazepine (n = 3), perampanel (n = 3), eslicarbazepine acetate (n = 2), valproic acid (n = 2), lamotrigine (n = 1), and oxcarbazepine (n = 1).
Table 2.
Baseline characteristics of participants according to the switch from levetiracetam
| Characteristics | No LEV switch (n = 23) | LEV switch (n = 21) | p value |
|---|---|---|---|
| Male sex | 14 (60.9) | 12 (57.1) | 0.802 |
| Age, years | 58 [39–78] | 65 [53–73] | 0.716 |
| Duration of epilepsy, years | 13 [3–23] | 6 [1–11] | 0.082 |
| Type of seizure | 0.378 | ||
| Focal onset | 12 (52.2) | 14 (66.7) | |
| Focal to bilateral tonic–clonic | 7 (30.4) | 6 (28.6) | |
| Focal onset and focal to bilateral tonic–clonica | 4 (17.4) | 1 (4.7) | |
| Aetiology | 0.450 | ||
| Structural | 12 (52.2) | 14 (66.7) | |
| Metabolic | 1 (4.3) | – | |
| Unknown | 10 (43.5) | 7 (33.3) | |
| Any psychiatric comorbidity | 7 (30.4) | 6 (28.6) | 0.892 |
| Number of prior ASMsb | 3 [1–5] | 1 [1, 2] | < 0.001 |
| Reason to introduce brivaracetam | < 0.001 | ||
| Lack of efficacy with other ASMs/LEV | 14 (60.9) | 2 (9.5) | |
| Adverse events with other ASMs/LEV | 3 (13.0) | 18 (85.7) | |
| Both | 6 (26.1) | 1 (4.8) | |
| Seizure freedom at baseline | 8 (34.8) | 9 (42.9) | 0.583 |
| Baseline monthly seizure frequencyc | 0.5 [0.3–0.8] | 0.4 [0.2–0.9] | 0.921 |
Data are median [IQR] for continuous variables and n (%) for categorical variables
ASM antiseizure medication, BRV brivaracetam, IQR interquartile range, LEV levetiracetam
aSubjects presenting both focal onset and focal to bilateral tonic–clonic seizures
bNumber of lifetime ASMs before starting treatment with BRV monotherapy
cBased on the number of seizures during the 6 months before starting BRV monotherapy in non-seizure-free subjects
All participants had 6-month follow-up and 12-month data were available for 31 (70.5%) subjects. Within the first 6 months, BRV was discontinued by three subjects, and one ASM was added to BRV in two other cases. The retention rate of BRV monotherapy was 88.6% (39/44) at 6 months and 83.9% (26/31) at 12-month follow-up. The overall rates of seizure freedom were 72.7% (32/44) in subjects with 6-month follow-up and 58.1% (18/31) in subjects with 12-month follow-up. The median maintenance dosage of BRV monotherapy was 150 (100–200) mg/day at 6 months and 125 (100–200) mg/day in subjects with 12-month follow-up. Sustained seizure freedom was observed in 18 out of 31 (58.1%) participants with 12-month follow-up. During the follow-up, seizures occurred in 2 out of the 17 (11.8%) subjects who were seizure free at baseline.
Adverse events were recorded in 6/44 (13.6%) participants and led to BRV discontinuation in 2/44 (4.6%) cases. The reported AEs were somnolence (n = 3), fatigue (n = 2), and irritability (n = 1); no serious AEs were experienced. The AEs leading to discontinuation of BRV monotherapy were somnolence (n = 1) and irritability (n = 1); both subjects were receiving BRV at the daily dosage of 200 mg.
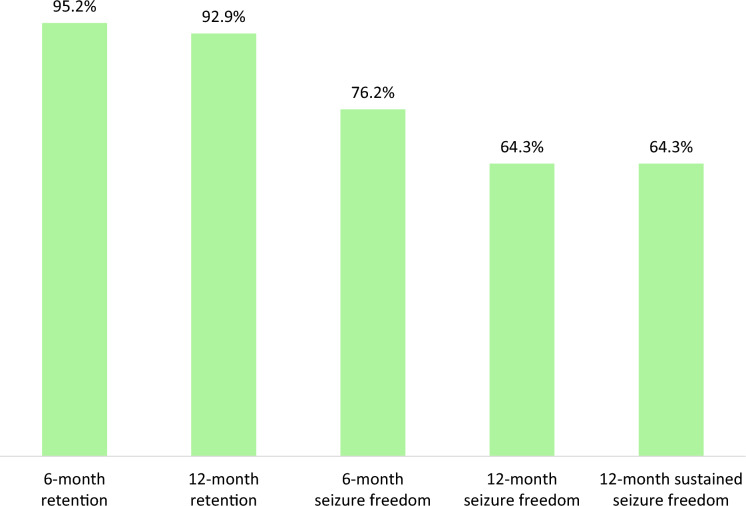
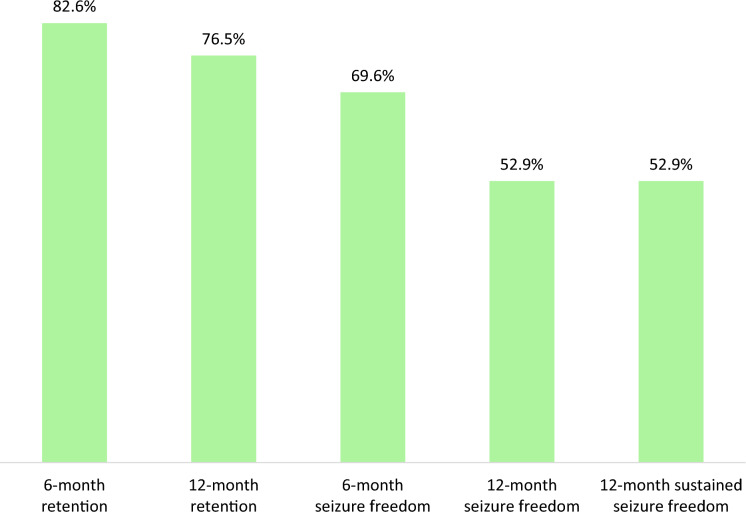
The rates of treatment retention and seizure freedom at 6 and 12 months and sustained seizure freedom were higher among people who switched from LEV (Fig. 1) compared to those who did not (Fig. 2), although statistical significance was not reached for any comparisons.
Fig. 1.
Effectiveness of brivaracetam monotherapy in subjects who switch from levetiracetam. Rates of retention of brivaracetam monotherapy, seizure freedom and sustained seizure freedom in subjects who switched (6-month follow-up, n = 21; 12-month follow-up, n = 14) from LEV (p > 0.05 for any comparisons). LEV levetiracetam
Fig. 2.
Effectiveness of brivaracetam monotherapy in subjects who did not switch from levetiracetam. Rates of retention of brivaracetam monotherapy, seizure freedom and sustained seizure freedom in subjects who did not switch from LEV (6-month follow-up, n = 23; 12-month follow-up, n = 17) (p > 0.05 for any comparisons). LEV levetiracetam
Discussion
This study described the 1-year experience with BRV administered in a cohort of people with focal epilepsy as monotherapy after the withdrawal of concomitant ASMs, including the direct switch from LEV. The findings suggested that monotherapy with BRV was effective and well tolerated as most of the participants remained on treatment throughout the follow-up. The retention rate was 87.8% at 6 months and slightly decreased to 83.9% at 12 months. In the overall study cohort, seizure freedom was observed in 72.7% and 58.1% of the subjects with 6- and 12-month follow-up available. All subjects who were seizure free at 12 months had no seizures from the beginning of BRV monotherapy throughout the follow-up.
A better response was found among people who switched from LEV to BRV, with higher rates of retention and freedom from seizures at both time points: nearly all people who switched from LEV continued treatment with BRV monotherapy, and about 65% reached 1-year sustained seizure freedom. Of note, poor tolerability was the most common reason to switch from LEV to BRV, suggesting that LEV was associated with a satisfactory seizure control, and participants who switched from LEV to BRV had fewer prior ASMs, suggesting that they could represent a less difficult-to-treat group. Overall, these data can indirectly support the studies exploring the add-on use of BRV and showing that AEs associated with LEV treatment can improve or resolve after the switch to BRV [10–12].
A low proportion of subjects treated with BRV monotherapy had AEs and treatment discontinuation due to tolerability issues was uncommon. Although the low incidence of AEs may be the consequence of the underreporting that can occur in retrospective studies, participants converted to monotherapy after progressive withdrawal of background ASMs represented a selected population who has been already on BRV treatment for time and, hence, less likely to have AEs. The recorded AEs were consistent with those observed in clinical trials [13, 14] and real-world studies [15–17] of adjunctive BRV, the documented ones being somnolence, fatigue, and irritability. No serious AEs or death occurred, and no new safety signals were identified.
So far, there are limited data available about the clinical experience with BRV when used as single-drug regimen. Two phase III, randomized, double-blind, multicentre, historical-controlled, conversion-to-monotherapy trials evaluated the efficacy, safety, and tolerability of conversion to BRV monotherapy in adults with uncontrolled focal seizures [18]. After randomization of 150 participants, the studies were terminated because of the confounding effects of a higher-than-expected discontinuation rate. In both studies, the cumulative exit rates were lower than the historical control threshold. In the sensitivity analysis, however, when censoring as a result of early withdrawal was limited to a maximum of 10% beyond which participants were considered to have met an exit criterion, the upper 95% confidence limit of the cumulative exit rate was above historical control. During the BRV monotherapy period, the incidence of AEs was 48.8% and the rate of treatment discontinuation due to AEs was 8.3%.
In the EXPERIENCE/EPD332, an international pooled analysis of individual patient records from multiple independent studies of patients with epilepsy initiating BRV in Australia, Europe, and the USA, 45 out of 1644 participants were on monotherapy at index [19]. They had a median epilepsy duration of 9 years and a median of 3 prior ASMs; no data were provided whether these cases were conversion to monotherapy, switch from LEV, or initial monotherapy. The median duration of exposure to BRV was 253 days, the BRV retention rate was 77.3% at 12 months, and tolerability was the most common reason for discontinuation. The 12-month responder and seizure freedom rates were 30.8% and 36.0% among 13 and 25 subjects for whom data were available, respectively, and the rate of continuous sustained freedom was 28.0%. The overall incidence of adverse events at 12 months was 3.8%.
In a retrospective, single-centre study performed at the Epilepsy Center Hessen, Germany, 93 subjects treated with BRV were included, and 12 of them received BRV monotherapy according to individual therapeutic decisions and medical reasons [20]. The median number of prior ASMs was 2, and the median time of observation was 4 months. Freedom from seizures was achieved in 9/12 (75%) subjects and treatment discontinuation occurred in 5/12 (41.7%) cases, with a median duration of therapy until discontinuation of 3.5 months. Two subjects (16.7%) were withdrawn from treatment because of behavioural AEs, and non-behavioural AEs accounted for 3 (25.0%) discontinuations. Adverse events were reported in 5/12 participants (41.7%), the most common being irritability and agitation. In two-thirds of people with LEV-related AEs at baseline, these events were reduced to a clinically and statistically significant extent during BRV treatment.
Despite the small number of participants, the population of this study was the largest cohort included in the few studies that so far have explored the effectiveness of BRV monotherapy in clinical practice. The study benefitted from participants receiving diagnosis, treatment, and monitoring at tertiary epilepsy centres, and from subgroup analyses according to the switch from LEV to BRV. Further, the broad inclusion criteria allowed participants to be representative of a real-world epilepsy population, heterogeneous in terms of age, aetiologies, comorbidities, and previous treatments, including subjects who are not typically evaluated in trials with more rigid entry criteria. Some limits need to be also acknowledged when interpreting the findings, including the retrospective and open-label study design, which may have introduced potential sources of selection bias and underreporting of AEs, and the lack of a comparison group, which prevented assessment of the comparative effectiveness of BRV with other ASMs. As a result of the descriptive nature of the study, no formal calculation of the sample size was performed. The small sample size also limited the possibility to perform subgroup analyses according to the baseline characteristics of participants, to run inferential statistics to identify potential association between the study outcomes and clinical variables, and to explore correlations of BRV dosage with AEs leading to stoppage. Furthermore, no details were collected about the strategies of conversion to monotherapy or switch from LEV, which were adopted at the sites by the treating physicians according to their own preferences and routine clinical practice, in the absence of any standardized protocols. The results from the analysis according to the switch from LEV need to be not overinterpreted, as the study was not primarily designed to address this question. Of note, no subjects receiving BRV as first-line monotherapy were included as per study protocol, and results cannot be extrapolated to this population. In addition, the mean age of the study cohort was skewed towards the elderly, thus limiting the generalizability of the findings to other age groups.
Conclusion
This retrospective, non-interventional chart review suggested that BRV may be a valuable treatment of focal seizures in people who decided to convert to BRV monotherapy in a real-life setting. Further studies, ideally prospective and based on larger cohorts, would be helpful to explore more extensively the clinical utility of BRV monotherapy and identify those people with focal seizures who may mostly benefit from the single-drug regimen.
Author Contributions
Simona Lattanzi designed and conceptualized the study, coordinated and supervised the data collection, carried out the data analyses, and drafted the manuscript. Nicoletta Foschi, Chiara Martellino, Daniela Audenino, Giovanni Boero, Paolo Bonanni, Edoardo Ferlazzo, Valentina Chiesa, Filippo Dainese, Marta Piccioli, Alessandra Ferrari, and Angelo Labate were involved in the acquisition of data. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript for submission and agree to be accountable for all aspects of the work. We thank Dr Bernardi Eugenio for his support in the data collection.
Funding
This work was supported by Ricerca Corrente 2023, funds for biomedical research of the Italian Ministry of Health. The journal's fee was waived.
Data Availability
The dataset generated during and/or analysed during the current study is available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
Simona Lattanzi has received speaker’s or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, Medscape, and UCB Pharma and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, Eisai, GW Pharmaceuticals, and Rapport Therapeutics outside the submitted work. Daniela Audenino has received speaker or consultancy fees from Angelini Pharma and UCB Pharma outside the submitted work. Paolo Bonanni has received speaker’s or consultancy fees from EISAI, Angelini, Jazz and Livanova and has served on advisory boards for BIAL, Eisai, Proveca outside the submitted work. Filippo Dainese has received speaker or consultancy fees from Angelini Pharma, Eisai and UCB Pharma outside the submitted work. Valentina Chiesa has received speaker and advisory board honoraria from Angelini Pharma, Eisai Pharma, GW, UCB Pharma outside the submitted work. Filippo Dainese has received speaker or consultancy fees from Angelini Pharma, Eisai and UCB Pharma outside the submitted work. Marta Piccioli has received speaker’s or consultancy fees from Angelini, EISAI, and UCB Pharma outside the submitted work. Nicoletta Foschi, Chiara Martellino, Giovanni Boero, Edoardo Ferlazzo, Alessandra Ferrari, and Angelo Labate have no conflicts of interest to declare.
Ethical Approval
The study was approved by the ethical committee of the Marche Polytechnic University, Ancona, Italy (ID CERM 176) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient or the legal representative for the collection and analysis of data and the dissemination of the results.
References
- 1.Klein P, Diaz A, Gasalla T, Whitesides J. A review of the pharmacology and clinical efficacy of brivaracetam. Clin Pharmacol. 2018;10:1–22. doi: 10.2147/CPAA.S114072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brivaracetam. Highlight of prescribing information. https://www.briviact.com/briviact-PI.pdf. Accessed Oct 2023.
- 3.Moseley BD, Chanteux H, Nicolas JM, Laloyaux C, Gidal B, Stockis A. A review of the drug-drug interactions of the antiepileptic drug brivaracetam. Epilepsy Res. 2020;163:106327. doi: 10.1016/j.eplepsyres.2020.106327. [DOI] [PubMed] [Google Scholar]
- 4.EMA. Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-clinical-investigation-medicinal-products-treatment-epileptic-disorders-revision-3_en.pdf. Accessed Oct 2023. [PubMed]
- 5.Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Lacosamide monotherapy for partial onset seizures. Seizure. 2015;27:71–74. doi: 10.1016/j.seizure.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 7.Lattanzi S, Ascoli M, Canafoglia L, et al. Sustained seizure freedom with adjunctive brivaracetam in patients with focal onset seizures. Epilepsia. 2022;63:e42–50. doi: 10.1111/epi.17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lattanzi S, Canafoglia L, Canevini MP, et al. Adjunctive brivaracetam and sustained seizure frequency reduction in very active focal epilepsy. Epilepsia. 2023;64:2922–2933. doi: 10.1111/epi.17740. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch M, Hintz M, Specht A, Schulze-Bonhage A. Tolerability, efficacy and retention rate of brivaracetam in patients previously treated with levetiracetam: a monocenter retrospective outcome analysis. Seizure. 2018;61:98–103. doi: 10.1016/j.seizure.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Yates SL, Fakhoury T, Liang W, Eckhardt K, Borghs S, D'Souza J. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav. 2015;52:165–168. doi: 10.1016/j.yebeh.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Steinhoff BJ, Klein P, Klitgaard H, et al. Behavioral adverse events with brivaracetam, levetiracetam, perampanel, and topiramate: a systematic review. Epilepsy Behav. 2021;118:107939. doi: 10.1016/j.yebeh.2021.107939. [DOI] [PubMed] [Google Scholar]
- 13.Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Brivaracetam add-on for refractory focal epilepsy: a systematic review and meta-analysis. Neurology. 2016;86:1344–1352. doi: 10.1212/WNL.0000000000002545. [DOI] [PubMed] [Google Scholar]
- 14.Lattanzi S, Trinka E, Zaccara G, et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. 2022;82:199–218. doi: 10.1007/s40265-021-01661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lattanzi S, Canafoglia L, Canevini MP, et al. Adjunctive brivaracetam in focal epilepsy: real-world evidence from the BRIVAracetam add-on First Italian netwoRk STudy (BRIVAFIRST) CNS Drugs. 2021;35:1289–1301. doi: 10.1007/s40263-021-00856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattanzi S, Canafoglia L, Canevini MP, et al. Brivaracetam as early add-on treatment in patients with focal seizures: a retrospective, multicenter real-world study. Neurol Ther. 2022;11:1789–1804. doi: 10.1007/s40120-022-00402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villanueva V, López-González FJ, Mauri JA, et al. BRIVA-LIFE—a multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta Neurol Scand. 2019;139:360–368. doi: 10.1111/ane.13059. [DOI] [PubMed] [Google Scholar]
- 18.Arnold S, Badalamenti V, Diaz A, et al. Conversion to brivaracetam monotherapy for the treatment of patients with focal seizures: two double-blind, randomized, multicenter, historical control, phase III studies. Epilepsy Res. 2018;141:73–82. doi: 10.1016/j.eplepsyres.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Villanueva V, Laloyaux C, D'Souza W, et al. Effectiveness and tolerability of 12-month brivaracetam in the real world: EXPERIENCE, an international pooled analysis of individual patient records. CNS Drugs. 2023;37:819–835. doi: 10.1007/s40263-023-01033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zahnert F, Krause K, Immisch I, et al. Brivaracetam in the treatment of patients with epilepsy-first clinical experiences. Front Neurol. 2018;9:38. doi: 10.3389/fneur.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during and/or analysed during the current study is available from the corresponding author upon reasonable request.