Fig. 3. Comparison the immunogenicity of the designed different chimeric VLPs, including HBcAg_MIR-II, WHcAg_MIR-II, GSHcAg_MIR-II, AGSHcAg_MIR-II, BTHcAg_MIR-II, and RBHcAg_MIR-II.
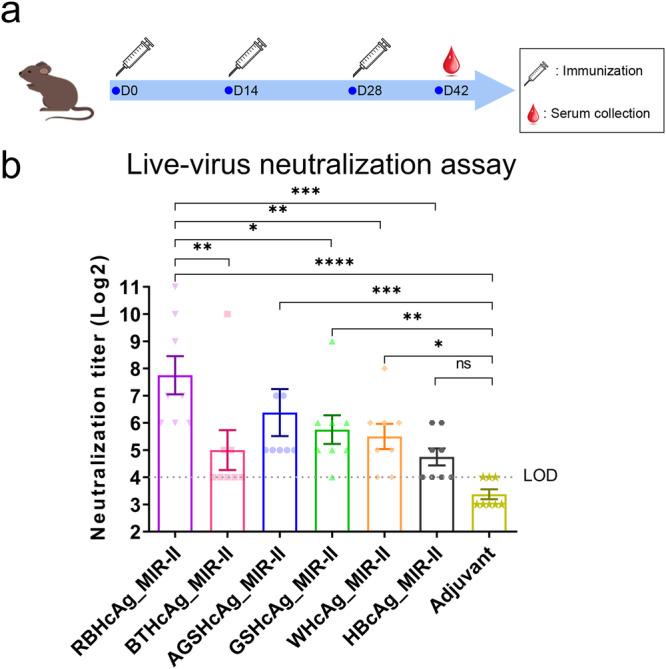
BALB/c mice (n = 8 per group) were immunized intraperitoneally on days 0, 14, and 28 with each chimeric VLP plus MF59-like adjuvant. Each dose contained 30 μg antigen (150 μl) and 150 μl adjuvant. Another group of mice was administered adjuvant only as a control. On day 14 after completion of the vaccination, neutralizing antibody titers in the serum of the immunized mice were measured using a live-virus neutralization assay. a Timeline of the mouse immunization and serum collection. b Live-virus neutralizing antibody titers in the serum of the mice immunized with different chimeric VLPs that display the RSV F epitope II. The quantification limit of the live-virus neutralization assay was 16, and the titer value below the limit of detection (LOD) was set to 8. Data are presented as mean ± SEM. The statistical significance of the difference between different groups was determined by One-way ANOVA with the LSD t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns not significant.
