Figure 2.
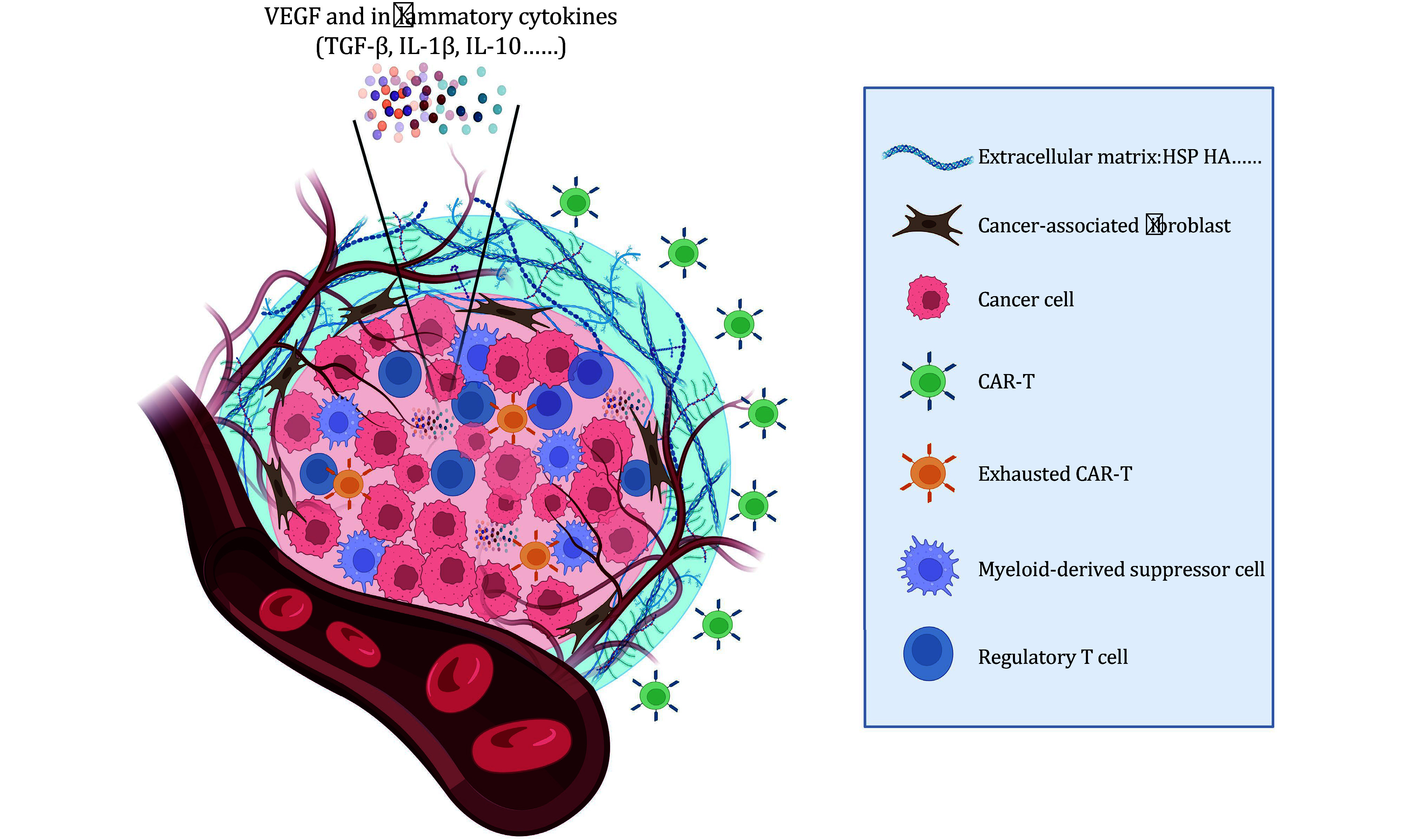
Schematic diagram of the solid tumor microenvironment. TME plays a pivotal role in limiting the efficacy of CAR-T therapy for solid tumors. The TME encompasses a complex network of interactions and conditions within and around the tumor, creating a hostile environment that hinders eradication. Several key factors contribute to this phenomenon. First, the TME consists of immune-suppressive cells, such as regulatory T cells and myeloid-derived suppressor cells. These cells impede the immune response against the tumor, thereby compromising the effectiveness of CAR-T-cell therapy. Additionally, hypoxia, characterized by oxygen deprivation, triggers the expression of VEGF. This leads to the abnormal formation of blood vessels through angiogenesis within the TME. Consequently, there is an inadequate supply of essential nutrients, ultimately compromising immune cell function. Moreover, cancer-associated fibroblasts present in the TME play a dual role. They not only promote tumor growth and invasion but also generate an extracellular matrix that obstructs the infiltration and activity of CAR-T cells. Furthermore, the release of inflammatory cytokines, including TGF-β, IL-1β, and IL-10, creates an inflammatory milieu within the TME that further supports tumor progression. These cumulative challenges posed by the hostile TME underscore the need for innovative approaches to overcome these barriers and enhance treatment outcomes in CAR-T therapy for solid tumors
