Figure 3. Loss of NPY in sympathetic neurons does not affect neuron survival and target innervation but results in reduced soma size.
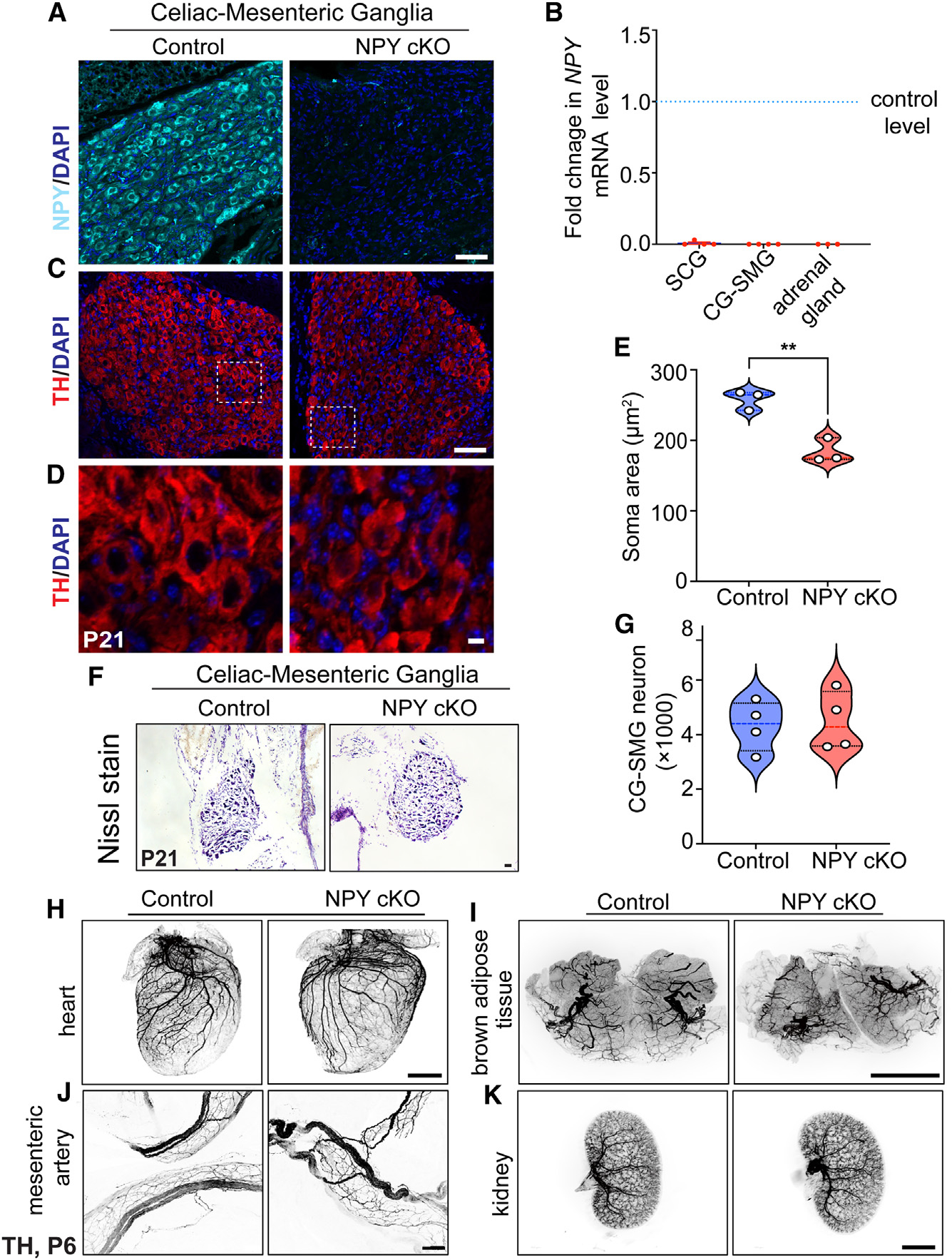
(A) Immunostaining shows a marked reduction in NPY protein in CG-SMGs from NPY cKO mice compared to control mice. Scale bar, 50 μm.
(B) qPCR analyses show a loss of Npy transcript in SCGs, CG-SMGs, and adrenal glands from NPY cKO mice. Results are means ± SEM from n = 4 animals per genotype for SCGs and CG-SMGs and n = 3 animals per genotype for adrenal glands.
(C and D) TH expression appears unaffected by NPY loss as shown by immunofluorescence.
(D) shows higher-magnification views of the insets in (C). Scale bars, 50 μm for (C) and 5 μm for (D).
(E) Quantification of soma areas from CG-SMG tissue sections shows that neuronal soma size is reduced in NPY cKO sympathetic ganglia. Results are means ± SEM from n = 3 mice per genotype. **p < 0.01; unpaired t test.
(F) Nissl staining of CG-SMGs in control and NPY cKO mice. Scale bar, 50 μm.
(G) Quantification of Nissl-stained neurons shows that neuron numbers are comparable between control and mutant mice. Results are means ± SEM from n = 4 per genotype.
(H–K) Whole-organ TH immunostaining imaged by light-sheet microscopy shows that sympathetic axon innervation of target tissues including the heart, BAT, mesenteric arteries, and kidneys is comparable between NPY cKO and control mice. Scale bars, mesenteric arteries: 50 μm; heart and kidney: 800 μm; and BAT: 1,500 μm.
