Fig. 2.
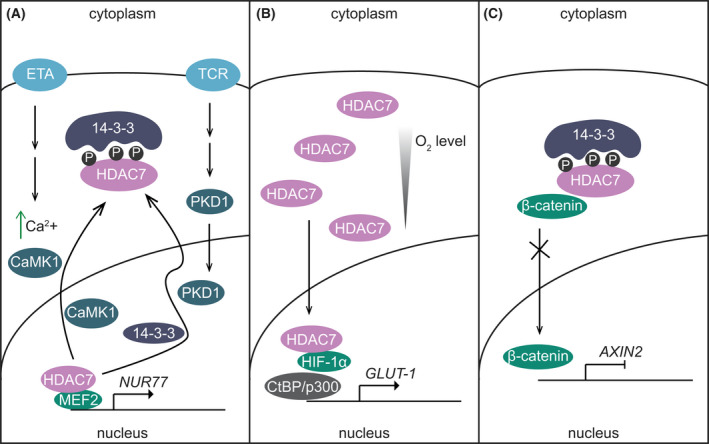
Subcellular localisation of HDAC7 results in distinct biological functions. (A) Nuclear export of HDAC7 enables inducible gene expression, via its action as a transcriptional derepressor. In response to increased Ca2+ concentrations or upon TCR activation, nuclear HDAC7 is phosphorylated by CaMK1 or PKD1, respectively, resulting in its nuclear export. Cytoplasmic HDAC7 binds to 14‐3‐3 at phosphorylated residues, with the nuclear export of HDAC7 enabling derepression of MEF2‐regulated genes such as NR4A1 (NUR77) in T cells and other cell types. (B) Nuclear import of HDAC7 results in inducible gene expression, via its action as a transcriptional activator. Under hypoxia, HDAC7 shuttles into the nucleus and binds to HIF‐1α and CBP/p300, initiating expression of HIF‐1α target genes such as SLC2A1 (GLUT1) in HEK293 cells. (C) The cytoplasmic HDAC7/14‐3‐3 complex retains β‐catenin in the cytoplasm, preventing its translocation into the nucleus, thus limiting expression of β‐catenin‐dependent genes such as AXIN2. Cytoplasmic functions of HDAC7 have been observed in multiple cell types, for example HUVEC and macrophages. CaMK1, calcium/calmodulin‐dependent kinase 1; ETA, endothelin receptor A; HIF‐1α, hypoxia‐inducible factor 1‐α; MEF2, myocyte enhancer factor‐2; PKD1, protein kinase D1; TCR, T cell receptor.
