Abstract
Objective
To define the association between change in body mass index (BMI) and the incidence and progression of structural defects of hip osteoarthritis as assessed by radiography.
Methods
We used data from 2 independent cohort studies: the Osteoarthritis Initiative (OAI) and the Cohort Hip and Cohort Knee (CHECK) study. Our exposure was change in BMI from baseline to 4–5 years’ follow‐up. Our outcomes were the incidence and progression of structural defects of hip osteoarthritis as assessed using a modified Croft grade in OAI and the Kellgren/Lawrence grade in the CHECK study. To study incidence, we created incidence cohorts of hips without definite overall structural defects at baseline (i.e., grade <2) and then investigated the odds of hips having definite overall structural defects at follow‐up (i.e., grade ≥2). To study progression, we created progression cohorts of hips with definite overall structural defects at baseline (i.e., grade ≥2) and then investigated the odds of having a grade increase of ≥1 from baseline to follow‐up.
Results
There was a total of 5,896 and 1,377 hips in the incidence cohorts, and 303 and 129 hips in the progression cohorts for the OAI and CHECK study, respectively. Change in BMI (decrease or increase) was not associated with any change in odds of the incidence or progression of definite structural defects of hip osteoarthritis in either the OAI or CHECK cohorts.
Conclusion
Weight loss may not be an effective strategy for preventing, slowing, or delaying the structural defects of hip osteoarthritis over 4–5 years.
INTRODUCTION
Hip osteoarthritis is an increasingly common and disabling degenerative joint disease (1). The prevalence of hip osteoarthritis has increased in almost all countries in the past 30 years, with the global age‐standardized prevalence increasing from 17.02 per 100,000 persons in 1990 to 18.70 per 100,000 persons in 2019 (2). In terms of managing osteoarthritis, guidelines from around the world recommend weight loss in individuals with overweight and obesity (3, 4, 5, 6, 7, 8). However, the recommendation of weight loss for hip osteoarthritis is based on research in knee osteoarthritis, as there is a paucity of research in the effects of weight loss on hip osteoarthritis (9).
SIGNIFICANCE & INNOVATIONS.
Weight loss may not be an effective strategy for preventing, slowing, or delaying the structural defects of hip osteoarthritis over 4–5 years.
Most guidelines recommend weight loss for hip osteoarthritis based on research on knee osteoarthritis, but there is no indication that this could be of benefit for hip osteoarthritis.
In light of this gap in knowledge, in this study we performed analyses to define the relationship between weight loss and hip osteoarthritis using data from 2 prospective cohort studies: the Osteoarthritis Initiative (OAI) (10) from the US, and the Cohort Hip and Cohort Knee (CHECK) (11) study from the Netherlands.
MATERIALS AND METHODS
Data sources and ethics
The data used in this study were obtained from the publicly available databases in the 2 cohort studies mentioned above. The OAI cohort consisted of participants with or at risk of knee osteoarthritis, and the CHECK study cohort had participants with early symptomatic knee or hip osteoarthritis. Ethical approval for the original OAI and CHECK studies were obtained by those studies, including obtaining informed written consents from all participants.
Exposure
Our exposure of interest was change in body mass index (BMI; in kg/m2) from baseline to follow‐up (time points detailed below). We used change in BMI instead of change in weight because weight data was not available in the CHECK study.
Outcomes
Our outcomes of interest were the incidence and progression of structural defects of hip osteoarthritis, as assessed by radiography. We used radiographic data from baseline and 4 years’ follow‐up from the OAI, and from baseline and 5 years’ follow‐up from the CHECK study. The OAI had no hip radiographic data at any other time points beyond 4 years. The CHECK study did not have radiographic data at 4 years; therefore, the 5‐year data were used.
We investigated the structural defects of hip osteoarthritis in 2 ways. In the first way, we investigated overall structural defects of hip osteoarthritis as assessed by radiography, scored using a modified Croft grade (12) in the OAI and by the Kellgren/Lawrence grade (13, 14) in the CHECK study. As both grades use the same grading system (i.e., range 0–4), we hereafter refer to them as “overall grade.” An overall grade of 0 indicates no hip osteoarthritis, an overall grade of 1 indicates possible hip osteoarthritis, and an overall grade of 2, 3, and 4 indicate mild, moderate, and severe hip osteoarthritis, respectively. For the purposes of this study, we refer to a hip with an overall grade of ≥2 as being with definite overall structural defects of hip osteoarthritis, and a hip with an overall grade of <2 as being without definite overall structural defects of hip osteoarthritis.
In addition to investigating overall structural defects of hip osteoarthritis, the second way in which we investigated the structural defects of hip osteoarthritis was to investigate defects of 9 individual structural features of the hip. The grades for these 9 individual structural features of the hip ranged from 0 to 3, and we hereafter refer to these as “individual grades.” These 9 individual structural features were joint space narrowing (JSN) in 2 locations (lateral and medial); osteophytes in 4 locations (acetabular superior; acetabular inferior; femoral superior; and femoral inferior); cysts in 1 location (acetabular subchondral); sclerosis in 1 location (femoral subchondral); and deformity in 1 location (femoral head). In addition to the individual grade for osteophytes in the 4 locations mentioned above, we investigated the sum of the individual grades for osteophytes in all 4 locations. We did this to increase power to detect any association between change in BMI and osteophytes, as small osteophytes in some locations may be difficult to detect (15). The reason why we investigated these individual structural features, in addition to overall structural defects of hip osteoarthritis (i.e., using the overall grade, as described above) was to provide insight into potential mechanisms for any associations between change in BMI and overall grade. Additionally, some individual features, for example, femoral osteophytes, alone can also be a valid indicator for structural defects of hip osteoarthritis (15, 16).
To study incidence of definite overall structural defects of hip osteoarthritis, we created incidence cohorts (1 for each of the OAI and CHECK study) of hips without definite overall structural defects of hip osteoarthritis at baseline (i.e., hips with an overall grade of <2). Incidence was defined as a hip in an incidence cohort moving from an overall grade of <2 at baseline to an overall grade of ≥2 at follow‐up.
To study progression of definite overall structural defects of hip osteoarthritis, we created progression cohorts (1 for each of the OAI and CHECK study) of hips with definite overall structural defects at baseline (i.e., hips with an overall grade of ≥2). Progression was defined as a hip in a progression cohort moving from an overall grade of 2 or 3 at baseline to an overall grade of ≥1 grades greater at follow‐up.
To study changes in the 9 individual structural features, we considered hips in both the incidence and progression cohorts in which the individual grade had increased from baseline by ≥1 individual grades. To study changes in the sum of the individual grades for osteophytes in all 4 locations, we considered hips in the incidence and progression cohorts in which the sum had increased from baseline by ≥2 individual grades (16). Because the incidence and progression cohorts were defined based on definite overall structural defects of the hip as determined by overall grade at baseline, and not based on individual structural features of the hip as determined by individual grade at baseline, an increase by ≥1 individual grades within the incidence or progression cohorts (or by ≥2 for the sum of the individual grades for osteophytes in all 4 locations) was neither exclusively incidence nor progression, and therefore, we referred to it nonspecifically as degeneration of individual structural features.
Selection of participants and hips
As outlined above, we created 2 incidence cohorts and 2 progression cohorts (i.e., the OAI incidence cohort, the CHECK incidence cohort, the OAI progression cohort, and the CHECK progression cohort). These were created by first applying selection criteria at the level of the participant and then at the level of the hip (Figure 1). Specifically, we excluded participants who had what was termed “cancer (other than skin cancer, leukemia or lymphoma)” in the OAI and who had what was termed “malignant disease or cancer” in the CHECK study at baseline or during follow‐up, those who were in the underweight category for BMI (<18.5 kg/m2) at baseline or during follow‐up, and those who had no data for BMI at baseline (Figure 1). After we applied these participant‐level exclusion criteria, there were 4,406 participants (8,812 hips) and 907 participants (1,814 hips) remaining in the OAI and CHECK cohorts, respectively (Figure 1). Of these 8,812 and 1,814 hips, we excluded hips that had been replaced prior to baseline (77 hips in the OAI cohort, and none in the CHECK cohort) and then sorted the remaining hips into 4 groups that formed the basis of our 4 study cohorts. The 2 incidence cohorts consisted only of hips without definite overall structural defects of hip osteoarthritis (i.e., hips with an overall grade of <2) at baseline. There were 5,896 hips from 3,053 participants in the OAI incidence cohort, and 1,377 hips from 743 participants in the CHECK incidence cohort. The 2 progression cohorts consisted of only hips with definite overall structural defects of hip osteoarthritis (i.e., hips with an overall grade of ≥2) at baseline. There were 303 hips from 241 participants in the OAI progression cohort, and 129 hips from 108 participants in the CHECK progression cohort (Figure 1).
Figure 1.
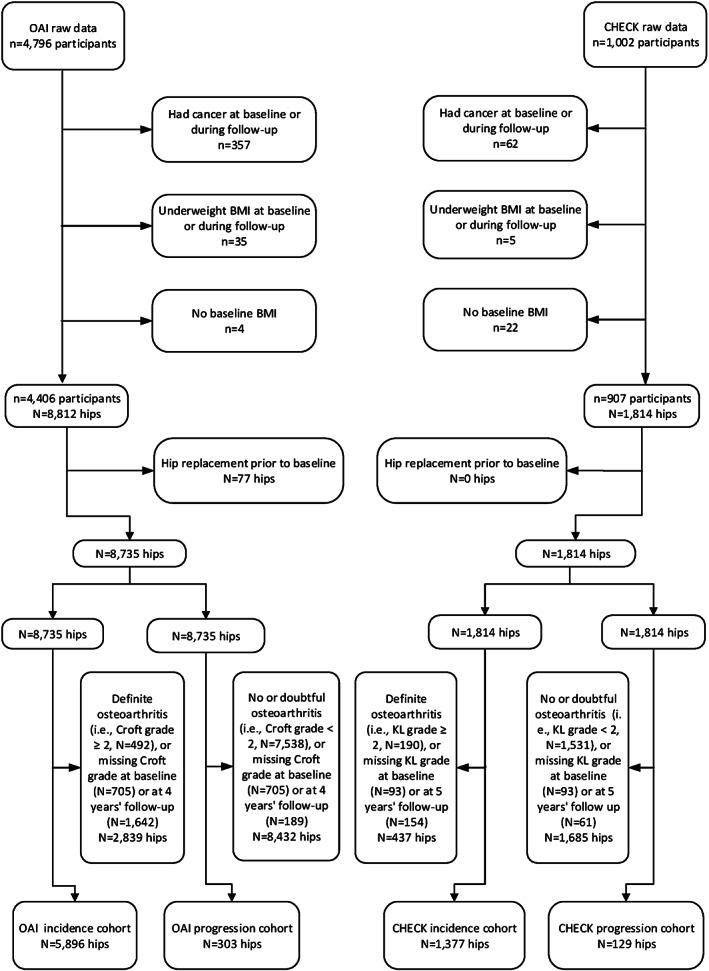
Selection of hips for our investigations of the incidence and progression of the definite overall structural defects of hip osteoarthritis or degeneration of individual structural features of the hip. BMI = body mass index; CHECK = Cohort Hip and Cohort Knee (study); KL = Kellgren/Lawrence; OAI = Osteoarthritis Initiative.
Statistical analyses
For all of the following statistical analyses, we used Stata, version BE 17.0 for Windows. We set our threshold for statistical significance as a 2‐tailed P value of less than 0.05 for all statistical analyses, including investigation into interactions (details below).
We used generalized estimating equations with a logistic link function (i.e., logistic regression with clustering of both hips within individuals) (17) to investigate the association of change in BMI between baseline and 4–5 years’ follow‐up and the outcomes listed above. Univariate (unadjusted) and multivariable (adjusted) analyses were performed. All of the multivariable analyses were adjusted for age at baseline, sex, and BMI at baseline, as these 3 variables are known to influence osteoarthritis outcomes. We also investigated the possibility of interactions between each of these 3 variables and change in BMI between baseline and 4–5 years’ follow‐up in the models that used overall grades as outcomes. We decided a priori that if there was a significant interaction then we would perform subgroup analyses, but no significant interactions (as indicated by a P value less than 0.05) were observed.
For all continuous variables in our analyses (i.e., age at baseline, BMI at baseline, and change in BMI), we tested the assumption of linearity using the Box‐Tidwell method (18). None of the continuous variables in this study violated the assumption of linearity.
Sensitivity analyses
We performed 4 types of sensitivity analyses. Note that these sensitivity analyses did not include any analyses in which missing data had been imputed because there were only 0.6% and 1.0% of follow‐up BMI data that was missing from the 4,406 and 907 participants we selected from the OAI and CHECK cohorts, respectively, and this level of missingness is considered inconsequential (19).
Our first type of sensitivity analysis aimed to determine whether the results for incidence of definite overall structural defects of hip osteoarthritis were different if we investigated only hips that had pain in addition to definite overall structural defects of hip osteoarthritis at follow‐up in an incidence cohort (hereafter referred to as “incidence of symptomatic hip osteoarthritis”). We only performed this sensitivity analysis in the incidence cohorts (both OAI and CHECK) and not in the progression cohorts. We did this sensitivity analysis because although a large proportion of patients with definite overall structural defects of hip osteoarthritis do not have hip pain (16), individuals with hip osteoarthritis seek medical help for their hip pain (16). Incidence of symptomatic hip osteoarthritis was defined as incidence of definite overall structural defects of hip osteoarthritis as defined above (i.e., moving from an overall grade of 0 or 1 at baseline to an overall grade of ≥2 at follow‐up in an incidence cohort) with the presence of pain at follow‐up. Pain was defined in 2 different ways in the OAI: the presence of any hip pain in the 12 months before follow‐up (“any hip pain”); and hip pain on more than one‐half of the days of a month in the 12 months before follow‐up (“frequent hip pain”). In the CHECK study, however, the only available data on pain concerned whether or not the participant had the presence of hip pain at the time of the follow‐up visit, and this was termed “presence of hip pain at examination.”
Our second type of sensitivity analysis aimed to determine whether adjusting our multivariable analyses for BMI at baseline may have altered the results, as it has been suggested that adjusting for a baseline variable (i.e., BMI in this case) can introduce bias in an analysis in which that same variable is used as or within the exposure variable (i.e., change in BMI from baseline to follow‐up) by increasing the risk for Type I errors (20). Thus, we conducted multivariable analyses by adjusting only for age at baseline and sex (instead of adjusting for age at baseline, sex, and BMI at baseline as in our primary analyses) and then compared the results with those of our primary analyses.
Our third type of sensitivity analyses aimed to assess the possible impact of including hips with possible hip osteoarthritis at baseline (i.e., overall grade = 1) in our incidence cohorts on our estimates of incidence of definite overall structural defects of hip osteoarthritis. We performed this sensitivity analysis in the incidence cohorts only. As a reminder, the incidence cohorts consisted of hips without definite overall structural defects of hip osteoarthritis at baseline (i.e., an overall grade of <2). It is possible that a hip with an overall grade of 1 might in fact have definite osteoarthritis, and this would then affect our estimates of the incidence of osteoarthritis. Therefore, we performed a sensitivity analysis by limiting the incidence cohorts to only those hips that had no hip osteoarthritis at baseline (i.e., an overall grade of 0) and compared the results with those of our primary analyses.
Our fourth type of sensitivity analysis aimed to determine whether our results were sensitive to overall grade of hip osteoarthritis at baseline. In this sensitivity analysis, we conducted multivariable analyses by additionally adjusting for overall grade at baseline (in addition to adjusting for age at baseline, sex, and BMI at baseline, as in our primary analyses) and then compared the results with those of our primary analyses. We have also tested the interaction of overall grade at baseline with change in BMI in this sensitivity analysis.
RESULTS
The following outlines the characteristics of the OAI and CHECK incidence and progression cohorts. It is noteworthy that the rates of incidence and progression of the overall structural defects of hip osteoarthritis and of degeneration of individual structural features of hip osteoarthritis were lower in the OAI than in the CHECK study (to be outlined below). The reason for this difference is that the OAI consisted of patients with or at risk of knee osteoarthritis, whereas the CHECK study consisted of patients with early osteoarthritis‐related complaints of hip and/or knee (21).
Characteristics of the OAI and CHECK incidence cohorts
Of the 5,896 hips in the OAI incidence cohort (i.e., hips with an overall grade of 0 or 1 at baseline), by 4 years’ follow‐up, 85 (1.44%) had definite overall structural defects of hip osteoarthritis (i.e., an overall grade of ≥2), while of the 1,377 hips in the CHECK incidence cohort, by 5 years’ follow‐up, 179 (13.00%) had definite overall structural defects of hip osteoarthritis (Table 1). The number of hips with degeneration of individual structural features ranged from 1 to 170 (0.02–2.88%) in the OAI incidence cohort and 3 to 357 (0.23–26.19%) in the CHECK incidence cohort (Table 1).
Table 1.
The number of cases of incidence and progression of definite overall structural defects of hip osteoarthritis (OA) and degeneration of individual structural features of the hip during 4–5 years’ follow‐up in each cohort*
| OAI | CHECK | |||
|---|---|---|---|---|
| Incidence cohort | Progression cohort | Incidence cohort | Progression cohort | |
| Participants, no. | 3,053 | 241 | 743 | 108 |
| Hips, no. | 5,896 | 303 | 1,377 | 129 |
| Overall structural defects of hip OA | ||||
| Total | 85 (1.44) | 0 (0.00) | 179 (13.00) | 8 (6.20) |
| Overall grade = 0 at baseline | 18 (0.30) | 0 (0.0) | 77 (5.6) | 8 (6.20) |
| Overall grade = 1 at baseline | 67 (1.14) | 0 (0.0) | 102 (7.4) | 0 (0.0) |
| Overall grade = 2 at baseline | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Overall grade = 3 at baseline | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Overall grade = 4 at baseline | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Degeneration of individual structural features of the hip | ||||
| Joint space narrowing lateral | 91 (1.54) | 38 (12.62) | 129 (9.39) | 11 (8.53) |
| Joint space narrowing medial | 170 (2.88) | 48 (15.89) | 260 (18.92) | 16 (12.40) |
| Osteophytes acetabular superior | 74 (1.26) | 27 (8.94) | 386 (28.20) | 38 (29.46) |
| Osteophytes acetabular inferior | 25 (0.42) | 17 (5.67) | 97 (7.43) | 16 (14.81) |
| Osteophytes femoral superior | 114 (1.93) | 30 (9.97) | 357 (26.19) | 40 (31.25) |
| Osteophytes femoral inferior | 25 (0.42) | 26 (8.64) | 179 (14.16) | 25 (23.58) |
| Sum of osteophyte scores | 39 (0.66) | 28 (9.33) | 244 (19.73) | 29 (30.53) |
| Subchondral cysts acetabular | 6 (0.10) | 8 (2.65) | 3 (0.23) | 1 (0.83) |
| Subchondral sclerosis femoral | 3 (0.05) | 12 (3.97) | 10 (0.77) | 4 (3.31) |
| Femoral head deformity | 1 (0.02) | 3 (0.99) | 69 (5.28) | 3 (2.48) |
Values are the number (%) unless indicated otherwise. The percentage calculations are based on complete case (i.e., excluding missing values). The 2 incidence cohorts consisted only of hips without definite overall structural defects of hip OA (i.e., an overall grade of <2) at baseline. The 2 progression cohorts consisted of only hips with definite overall structural defects of hip OA (i.e., an overall grade of ≥2) at baseline. CHECK = Cohort Hip and Cohort Knee (study); OAI = Osteoarthritis Initiative.
Compared to the age of those who had hips without definite overall structural defects of hip osteoarthritis at follow‐up (mean ± SD 60.4 ± 9.0 and 55.5 ± 5.2 years in the OAI and CHECK incidence cohorts, respectively), participants who had hips with definite overall structural defects of hip osteoarthritis at follow‐up were older (mean ± SD 64.6 ± 8.4 and 56.2 ± 5.2 years in the OAI and CHECK incidence cohorts, respectively) (Table 2). The groups that had hips with definite overall structural defects of hip osteoarthritis at follow‐up had a greater proportion of female than male participants in both OAI and CHECK (57.7% and 77.9%, respectively) (Table 2). Participants who had hips with definite overall structural defects of hip osteoarthritis at follow‐up had a similar BMI to those who did not in both OAI (mean ± SD 27.5 ± 4.3 versus 28.3 ± 4.5 kg/m2) and CHECK (26.6 ± 4.0 versus 26.2 ± 4.0 kg/m2) (Table 2). The mean ± SD follow‐up time was 4.0 ± 0.1 (range 3.5–4.3) years in the OAI incidence cohort. For the CHECK incidence cohort, there were no data for the date of follow‐up; therefore, we assumed 5 years of follow‐up time for those who had outcome data at 5 years’ follow‐up (Table 2).
Table 2.
Characteristics of participants in each incidence cohort stratified by incidence of definite overall structural defects of hip osteoarthritis (OA) during the 4–5 years’ follow‐up*
| Incidence of definite overall structural defects of hip OA in the OAI | Incidence of definite overall structural defects of hip OA in CHECK | |||||
|---|---|---|---|---|---|---|
| Yes | No | Total | Yes | No | Total | |
| Participants, no. | 82 | 3,026 | 3,053 | 150 | 672 | 743 |
| Hips, no. | 85 | 5,811 | 5,896 | 179 | 1,198 | 1,377 |
| Age, years | 64.6 ± 8.4 | 60.4 ± 9.0 | 60.5 ± 9.0 | 56.2 ± 5.2 | 55.5 ± 5.2 | 55.6 ± 5.2 |
| Sex, no. (%) | ||||||
| Male | 27 (32.9) | 1,283 (42.4) | 1,291 (42.3) | 39 (26.0) | 126 (18.8) | 149 (20.1) |
| Female | 55 (67.1) | 1,743 (57.6) | 1,762 (57.7) | 111 (74.0) | 546 (81.2) | 594 (79.9) |
| BMI, kg/m2 | 27.5 ± 4.3 | 28.3 ± 4.5 | 28.3 ± 4.5 | 26.6 ± 4.0 | 26.2 ± 4.0 | 26.3 ± 3.9 |
| Follow‐up, years | 4.0 ± 0.1 | 4.0 ± 0.1 | 4.0 ± 0.1 | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 |
Values are the mean ± SD unless indicated otherwise. The percentage calculations are based on complete case (i.e., excluding missing values). The follow‐up (in years) for the CHECK study is assumed as 5 years due to lack of availability of data. BMI = body mass index; CHECK = Cohort Hip and Cohort Knee (study); OAI = Osteoarthritis Initiative.
The change in BMI from baseline to follow‐up was similar in the OAI and CHECK incidence cohorts (the mean ± SD and range were 0.11 ± 1.91 and –12.10 to 9.5 kg/m2, and 0.10 ± 2.06 and –12.00 to 18.00 kg/m2, respectively). Supplementary Figures 1A and B, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.25057, show the distribution of participants by change in BMI from baseline to follow‐up in the OAI and CHECK incidence cohorts, respectively.
Characteristics of the OAI and CHECK progression cohorts
Of the 303 hips in the OAI progression cohort (i.e., hips with an overall grade of ≥2 at baseline), there were no hips that had progressed by ≥1 overall grades in overall structural defects of hip osteoarthritis at 4 years’ follow‐up (Table 1), while of the 129 hips in the CHECK progression cohort, there were only 8 hips (6.2%) that had progressed by ≥1 overall grades in overall structural defects of hip osteoarthritis at 5 years’ follow‐up. Although there were no or only a small number of hips with progression in overall structural defects, there were larger numbers of hips with degeneration of individual structural features in the OAI and CHECK progression cohorts, ranging from 3 to 48 (0.99–15.89%) in the OAI and 1 to 40 (0.83–31.25%) in CHECK (Table 1).
Compared to the CHECK progression cohort, the OAI progression cohort was older (mean ± SD 63.2 ± 8.9 versus 57.2 ± 4.8 years), had a lower proportion of female participants (43.1% versus 63.9%), and had a higher BMI (mean ± SD 28.3 ± 4.4 versus 26.5 ± 3.3 kg/m2) (Table 3). The mean ± SD follow‐up time was 4.0 ± 0.1 (range 3.5–4.3) years in the OAI progression cohort. For the CHECK progression cohort, as for the CHECK incidence cohort, there were no data for date of follow‐up; therefore, we assumed 5 years of follow‐up time for those who had outcome data at 5 years’ follow‐up (Table 3).
Table 3.
Characteristics of participants in each progression cohort stratified by progression of definite overall structural defects of hip osteoarthritis (OA) during the 4–5 years’ follow‐up*
| Progression of definite overall structural defects of hip OA in the OAI | Progression of definite overall structural defects of hip OA in CHECK | |||||
|---|---|---|---|---|---|---|
| Yes | No | Total | Yes | No | Total | |
| Participants, no. | 0 | 241 | 241 | 7 | 103 | 108 |
| Hips, no. | 0 | 303 | 303 | 8 | 121 | 129 |
| Age, years | No obs. | 63.2 ± 8.9 | 63.2 ± 8.9 | 57.6 ± 1.9 | 57.1 ± 4.8 | 57.2 ± 4.8 |
| Sex, no. (%) | ||||||
| Male | No obs. | 137 (56.9) | 137 (56.9) | 2 (28.6) | 38 (36.9) | 39 (36.1) |
| Female | No obs. | 104 (43.1) | 104 (43.1) | 5 (71.4) | 65 (63.1) | 69 (63.9) |
| BMI, kg/m2 | No obs. | 28.3 ± 4.4 | 28.3 ± 4.4 | 25.0 ± 0.7 | 26.6 ± 3.4 | 26.5 ± 3.3 |
| Follow‐up, years | No obs. | 4.0 ± 0.1 | 4.0 ± 0.1 | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 |
Values are the mean ± SD unless indicated otherwise. The percentage calculations are based on complete case (i.e., excluding missing values). The follow‐up (in years) for the CHECK study is assumed as 5 years due to lack of availability of data. BMI = body mass index; CHECK = Cohort Hip and Cohort Knee (study); No obs. = no observations; OAI = Osteoarthritis Initiative.
The change in BMI from baseline to follow‐up was similar in the OAI and CHECK progression cohorts (the mean ± SD and range were –0.11 ± 1.66 and –5.90 to 4.10 kg/m2 in the OAI progression cohort, and 0.21 ± 2.24 and –5.00 to 18.00 kg/m2 in the CHECK progression cohort). Supplementary Figures 1C and D, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.25057, show the distribution of participants by change in BMI from baseline in the OAI and CHECK progression cohorts, respectively.
Incidence and progression of the overall structural defects of hip osteoarthritis over 4–5 years
Tables 4 and 5 show the estimates of the association of change in BMI between baseline and 4–5 years’ follow‐up and the odds in that time of the following: the incidence of definite overall structural defects of hip osteoarthritis as assessed by overall grade of hip osteoarthritis in both the OAI and CHECK incidence cohorts; and the progression of definite overall structural defects of hip osteoarthritis in the CHECK progression cohort. The reader is reminded that the association for progression cannot be determined in the OAI progression cohort, as there were no hips in that cohort that had progression of definite overall structural defects. In Tables 4 and 5, both unadjusted (univariate) and adjusted (multivariable) analyses are shown. There was no evidence in either the OAI or the CHECK study of association of change in BMI (decrease or increase) with the incidence or progression of definite overall structural defects of hip osteoarthritis (Tables 4 and 5), albeit progression could not be determined in the OAI as outlined above, and there were only 8 hips that had progression of definite overall structural defects in the CHECK study.
Table 4.
Association of change in body mass index (BMI) between baseline and 4–5 years’ follow‐up and the odds of incidence of definite overall structural defects of hip osteoarthritis (OA), as well as degeneration of individual structural features of the hip during the 4–5 years’ follow‐up, as shown in univariate and multivariable analyses*
| Outcome | OAI univariate analysis | OAI multivariable analysis† | CHECK univariate analysis | CHECK multivariable analysis† | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Incidence of definite overall structural defects of hip OA | 1.00 (0.89–1.12) | 0.99 | 1.01 (0.90–1.15) | 0.81 | 1.01 (0.93–1.10) | 0.77 | 1.03 (0.94–1.13) | 0.49 |
| Degeneration of individual structural features of the hip (as investigated in the incidence cohorts) | ||||||||
| Joint space narrowing lateral | 0.97 (0.87–1.09) | 0.64 | 0.98 (0.87–1.10) | 0.69 | 1.00 (0.91–1.10) | 0.96 | 1.00 (0.91–1.11) | 0.93 |
| Joint space narrowing medial | 1.03 (0.94–1.12) | 0.57 | 1.06 (0.97–1.16) | 0.23 | 1.00 (0.93–1.08) | 0.98 | 1.01 (0.93–1.09) | 0.87 |
| Osteophytes acetabular superior | 0.95 (0.84–1.07) | 0.40 | 0.95 (0.84–1.08) | 0.44 | 1.02 (0.96–1.09) | 0.47 | 1.04 (0.97–1.11) | 0.29 |
| Osteophytes acetabular inferior | 1.02 (0.82–1.27) | 0.87 | 1.03 (0.82–1.29) | 0.81 | 0.86 (0.77–0.95) | <0.01 | 0.85 (0.75–0.95) | <0.01 |
| Osteophytes femoral superior | 0.96 (0.87–1.06) | 0.42 | 0.97 (0.87–1.07) | 0.53 | 095 (0.89–1.01) | 0.11 | 0.93 (0.87–1.00) | 0.07 |
| Osteophytes femoral inferior | 1.04 (0.84–1.30) | 0.71 | 1.11 (0.87–1.41) | 0.40 | 1.00 (0.92–1.08) | 0.91 | 1.01 (0.93–1.10) | 0.77 |
| By sum of osteophyte scores | 1.14 (0.95–1.35) | 0.16 | 1.15 (0.96–1.38) | 0.13 | 0.97 (0.90–1.04) | 0.97 | 1.00 (0.92–1.08) | 0.90 |
| Subchondral cysts acetabular | 1.16 (0.76–1.76) | 0.49 | 1.21 (0.76–1.94) | 0.43 | 0.71 (0.51 0.99) | 0.04 | 0.70 (0.45–1.07) | 0.10 |
| Subchondral sclerosis femoral | 1.22 (0.68–2.16) | 0.50 | 1.22 (0.66–2.26) | 0.52 | 0.94 (0.67–1.33) | 0.74 | 0.80 (0.51–1.24) | 0.32 |
| Femoral head deformity | 1.44 (0.62–3.36) | 0.40 | 1.49 (0.61–3.62) | 0.38 | 1.08 (0.94–1.23) | 0.29 | 1.06 (0.92–1.23) | 0.43 |
The estimates are reported as point estimates of a 1 BMI–unit (kg/m2) increase from baseline to 4–5 years’ follow‐up. 95% CI = 95% confidence interval; CHECK = Cohort Hip and Cohort Knee (study); OAI = Osteoarthritis Initiative; OR = odds ratio.
The multivariable analysis was adjusted for age at baseline, sex, and BMI at baseline.
Table 5.
Association of change in body mass index (BMI) between baseline and 4–5 years’ follow‐up and the odds of progression of definite overall structural defects of hip osteoarthritis (OA), as well as degeneration of individual structural features of the hip, as shown in univariate and multivariable analyses*
| Outcome | OAI univariate analysis | OAI multivariable analysis† | CHECK univariate analysis | CHECK multivariable analysis† | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Progression of definite overall structural defects of hip OA | No observation | – | No observation | – | 0.85 (0.50–1.44) | 0.55 | 0.73 (0.38–1.41) | 0.35 |
| Degeneration of individual structural features of the hip (as investigated in the progression cohorts) | ||||||||
| Joint space narrowing lateral | 1.11 (0.90–1.37) | 0.33 | 1.11 (0.90–1.37) | 0.32 | 0.94 (0.66–1.35) | 0.75 | 0.95 (0.66–1.36) | 0.76 |
| Joint space narrowing medial | 0.85 (0.70–1.03) | 0.09 | 0.86 (0.71–1.04) | 0.12 | 0.88 (0.61–1.27) | 0.51 | 0.90 (0.61–1.32) | 0.58 |
| Osteophytes acetabular superior | 0.91 (0.71–1.17) | 0.47 | 0.92 (0.71–1.18) | 0.50 | 0.79 (0.59–1.06) | 0.11 | 0.77 (0.57–1.05) | 0.10 |
| Osteophytes acetabular inferior | 0.81 (0.61–1.08) | 0.15 | 0.82 (0.61–1.09) | 0.17 | 0.85 (0.60–1.21) | 0.36 | 0.85 (0.60–1.20) | 0.35 |
| Osteophytes femoral superior | 1.26 (0.98–1.61) | 0.07 | 1.27 (0.99–1.64) | 0.06 | 0.84 (0.65–1.09) | 0.19 | 0.85 (0.66–1.10) | 0.23 |
| Osteophytes femoral inferior | 0.89 (0.70–1.13) | 0.33 | 0.89 (0.70–1.13) | 0.33 | 1.10 (0.91–1.33) | 0.31 | 1.09 (0.90–1.32) | 0.39 |
| By sum of osteophyte scores | 1.10 (0.86–1.40) | 0.44 | 1.10 (0.87–1.41) | 0.42 | 0.94 (0.75–1.18) | 0.59 | 0.93 (0.74–1.17) | 0.54 |
| Subchondral cysts acetabular | 0.69 (0.46–1.03) | 0.07 | 0.65 (0.42–1.01) | 0.06 | 0.94 (0.28 3.19) | 0.92 | Insufficient observations | – |
| Subchondral sclerosis femoral | 0.93 (0.65–1.33) | 0.69 | 0.96 (0.67–1.36) | 0.80 | 0.94 (0.50–1.75) | 0.84 | 0.88 (0.44–1.77) | 0.72 |
| Femoral head deformity | 0.54 (0.29–1.00) | 0.05 | 0.56 (0.28–1.12) | 0.10 | 0.51 (0.20–1.31) | 0.16 | 0.41 (0.11–1.49) | 0.18 |
The estimates are reported as point estimates of a 1 BMI–unit (kg/m2) increase from baseline to 4–5 years’ follow‐up. 95% CI = 95% confidence interval; CHECK = Cohort Hip and Cohort Knee (study); OAI = Osteoarthritis Initiative; OR = odds ratio.
The multivariable analysis was adjusted for age at baseline, sex, and BMI at baseline.
Degeneration of individual structural features of the hip as assessed by radiography over 4–5 years
There was no association between change in BMI (decrease or increase) and the odds of degeneration of any of the 9 individual structural features of the hip (JSN in 2 locations [lateral and medial]; osteophytes in 4 locations [acetabular superior; acetabular inferior; femoral superior; and femoral inferior]; cysts in 1 location [acetabular subchondral]; sclerosis in 1 location [femoral subchondral]; and deformity in 1 location [femoral head]), nor in degeneration in the sum of the individual grades for osteophytes in all 4 locations, in any of the 4 cohorts (the incidence and progression cohorts from OAI and CHECK), with the exception of acetabular inferior osteophytes in the CHECK incidence cohort (Tables 4 and 5). The exception mentioned above showed an odds ratio (OR) of 0.85 with a 95% confidence interval (95% CI) of 0.75–0.95 in the multivariable analysis, implying that decrease in BMI is associated with increased odds of degeneration of this individual structural feature. Although there was no association of decrease in BMI with reduced odds of degeneration in any individual structural defects, some of the individual features, in particular cysts, sclerosis, and flat head deformity, had lower incident numbers; therefore, the null associations for these features can be considered inconclusive.
Results of sensitivity analyses
In our first type of sensitivity analysis, there were 45 (0.8%) and 25 (0.4%) of 5,896 hips in the OAI incidence cohort that had incident symptomatic hip osteoarthritis using the definitions of pain of “any hip pain” and “frequent hip pain,” respectively. In the CHECK incidence cohort, using the definition of “presence of hip pain at examination,” there were 71 (5.2%) of 1,377 hips that had incident symptomatic hip osteoarthritis. There was no association of change in BMI with the incidence of symptomatic hip osteoarthritis in either the OAI or the CHECK incidence cohort, and this is the same finding as in our primary analyses, in which our outcome of interest was the incidence of definite overall structural defects of hip osteoarthritis, which included hips that did and did not have pain at follow‐up. In this type of sensitivity analysis, the ORs in the OAI incidence cohort were 1.07 (95% CI 0.91–1.26) using the pain definition of “any pain” and 1.19 (95% CI 0.97–1.47) using the pain definition of “frequent pain.” The OR in the CHECK incidence cohort, using the pain definition of “presence of hip pain at examination” was 1.01 (95% CI 0.89–1.15). The results obtained from the other 3 types of sensitivity analyses (see sensitivity analyses in the Materials and Methods for details) gave results that were similar or the same as the results obtained from our primary analyses (results not shown).
DISCUSSION
This study showed that there was no association of change in BMI (decrease or increase) with odds of the incidence or progression of definite overall structural defects of hip osteoarthritis, nor with degeneration of individual structural features of the hip, in either the OAI or CHECK cohorts. Our findings thus suggest that weight loss may not be an effective intervention to prevent, slow, or delay the structural defects of hip osteoarthritis over 4–5 years.
One anomaly in our findings of null associations was the association between decrease in BMI and the increased odds of incidence of acetabular inferior osteophytes in the CHECK incidence cohort, suggesting a negative effect of decrease in BMI. Given that the only observed association for acetabular inferior osteophytes was in the CHECK incidence cohort but not in the OAI incidence cohort, nor in the CHECK progression cohort, and given previous research showing that acetabular osteophytes alone are not a reliable measure of structural defects in hip osteoarthritis (15, 16), this finding can be neglected.
This study adds to the finding that there is no evidence of benefit of weight loss for hip osteoarthritis in the literature. We know of 6 previously published studies that investigated the association of weight loss with hip osteoarthritis (22, 23, 24, 25, 26, 27). The first study (22), a systematic review, did not draw definitive conclusions on the positive influence of weight loss by bariatric surgery because the results from the 9 observational studies included in that systematic review were too limited or had uncertain and high bias (22). The second study, which investigated the association of weight loss with pain in hip osteoarthritis (23), was a preliminary study (n = 35 participants) that focused on the effect of exercise and weight loss over 8 months in patients with hip osteoarthritis and overweight or obesity. Although that study (23) found a 25.4% reduction in self‐reported hip pain after an average weight loss of 5% at the end of the 8 months, the lack of a control group and small sample size precludes any conclusion about the potential efficacy of weight loss for hip pain in this population. The third study (24), an observational study using OAI data (1 of the 2 data sources for the current study), also did not find an association between change in BMI and the development or resolution of hip pain, or the progression of the overall structural defects of hip osteoarthritis; nor the degeneration of an individual structural feature (i.e., JSN), nor total hip replacement, over 4 years. Our current study extends the findings from that third study (24) in several ways. First, the third study (24) treated change in BMI as a categorical variable, whereas we treated it as the continuous variable that it is. Treating continuous variables as categorical variables leads to loss of information, power, and efficiency in analyses (28). Second, while the third study (24) investigated the outcomes of progression of overall structural defects of hip osteoarthritis and degeneration of an individual structural feature (i.e., JSN), we additionally investigated the outcomes of incidence of overall structural defects of hip osteoarthritis and degeneration of 8 individual structural features besides JSN. Third, the third study (24) excluded participants who exhibited a weight change (loss or gain) of 3–5%, and this led to exclusion of >30% of participants from the OAI. Thus, in our study, with 30% more participants than the third study (24) in the OAI, as well as using another cohort (CHECK), we extended the findings from the third study by showing no association of change in BMI with the incidence or progression of overall structural defects in hip osteoarthritis, nor in the degeneration of individual structural features of the hip. The fourth (25), fifth (26), and sixth (27) studies investigated the association of weight change with the incidence of hip replacement over 5.2 years, 8 years, and 7–10 years, respectively. None of these 3 studies found an association of weight loss with hip replacement overall. While the fifth study, which used the OAI cohort data (26), found an association of weight change (loss or gain) with risk of hip replacement in a subgroup of patients with hip pain at baseline over 8 years (hazard ratio 1.03 [95% CI 1.01–1.05]), this finding is in contrast with the findings from a large multicohort study (27) that did not find any evidence for association of weight loss or gain with risk of hip replacement in participants followed up over a duration of 7–10 years from 3 independent cohort studies: the OAI and the CHECK study, which we use in this current study, and the Multicenter Osteoarthritis Study (MOST). In sum, looking at the findings from these 6 previously published studies (22, 23, 24, 25, 26, 27) and the current study, it seems that weight loss is not beneficial for hip osteoarthritis.
In light of the apparent lack of benefit of weight loss for hip osteoarthritis, it is interesting to note that weight loss is recommended for individuals with overweight and obesity in several guidelines around the world for the management of hip osteoarthritis (3, 4, 5, 6, 7, 8). An exception to this is the 2019 Osteoarthritis Research Society International guidelines (29), which cited the absence of clinical trials in hip osteoarthritis as a rationale for not recommending weight loss for hip osteoarthritis. The recommendation for weight loss in those guidelines (3, 4, 5, 6, 7, 8) is based on research on knee osteoarthritis. However, while there is evidence that weight loss is of benefit for knee osteoarthritis (30, 31), as well as having clear benefits for other aspects of health (notably cardiometabolic health) for those with overweight and obesity (32, 33), weight loss (intentional or unintentional) may carry health risks for some people, especially for older adults (34). For example, weight loss in older adults is associated with increased risk of hip fracture in female (35) and male (36) adults, as well as an increased risk of mortality (37) and higher risk of functional impairment and incident disability (38). This evidence of increased risk of certain health problems for some individuals, alongside the prevailing evidence of no benefit of weight loss for hip osteoarthritis, raises uncertainty about the recommendation of weight loss for hip osteoarthritis. Moreover, with now 7 publications, 6 previously published and this one, showing no association of weight loss with any apparent benefit for hip osteoarthritis, the value of doing a clinical trial investigating weight loss interventions for hip osteoarthritis is questionable.
Our study has several limitations. First, our study is an observational study; therefore, our findings are associative rather than causative. Second, there were likely latent confounders in our analyses that were not captured. Third, we used change in BMI between baseline and 4–5 years’ follow‐up, but BMI can fluctuate during that time, and our study did not capture these changes. Fourth, there were no events of progression of overall structural defects of hip osteoarthritis in the OAI, and there were only 8 (6.2%) of 129 hips in the CHECK cohort that had progression of overall structural defects. This limits our conclusions about the association between change in BMI and progression of overall structural defects. However, this limitation is mitigated by our finding of no evidence of the degeneration of any of the 9 individual structural features nor the sum of individual grades for osteophytes in all 4 locations that we investigated in the OAI and CHECK progression cohorts, in which we investigated the progression of overall structural defects.
Although the results of 3 of these 9 individual structural features, namely, cysts, sclerosis, and flat head deformity, may be considered inconclusive due to low incidence numbers, 6 of these individual structural features and the sum of the individual grades for osteophytes in all 4 locations can be considered conclusive. Last, the 2 cohorts used in this study were predominantly comprised of female and White participants; therefore, our findings have limited transferability beyond this specific population. To minimize the effect of these limitations, we used several strategies. First, we included both hips of each participant (where the selection criteria allowed) to avoid bias due to systematic exclusion of 1 hip from each participant. Second, participants in this study were followed up for a relatively long period of time (i.e., 4–5 years). Previous research shows that a minimum of 3 years’ follow‐up is required to detect the development of functional defects and pain in hip osteoarthritis (39). Therefore, 4–5 years’ follow‐up enabled a relatively large number of hips to have incidence of overall structural defects of hip osteoarthritis and degeneration of individual structural features, hence enabling a relatively more accurate estimation of the association between change in BMI and these outcomes.
In conclusion, this study showed no evidence of association between change in BMI and the incidence or progression of the overall structural defects of hip osteoarthritis, nor with the degeneration of individual structural features of the hip over 4–5 years. Thus, weight loss may not be an effective strategy for preventing, slowing, or delaying the structural defects of hip osteoarthritis over 4–5 years.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Mr. Salis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Salis, Sainsbury.
Acquisition of data
Salis.
Analysis and interpretation of data
Salis, Sainsbury.
Supporting information
Disclosure Form
Supplementary Figure 1: Histogram of BMI change
ACKNOWLEDGMENTS
Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
This article was prepared using an Osteoarthritis Initiative (OAI) public‐use data set, and its contents do not necessarily reflect the opinions or views of the OAI Study Investigators, the NIH, or the private funding partners of the OAI. The OAI data repository is housed within the National Institute of Mental Health Data Archive. The OAI is a public–private partnership between the NIH (contracts N01‐AR‐2‐2258, N01‐AR‐2‐2259, N01‐AR‐2‐2260, N01‐AR‐2‐2261, and N01‐AR‐2‐2262) and private funding partners (Merck Research Laboratories, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer, Inc.) and is conducted by the OAI Study Investigators. Private sector funding for the OAI is managed by the Foundation for the NIH. The authors of this article are not part of the OAI investigative team.
The Cohort Hip and Cohort Knee (CHECK) study is funded by the Dutch Arthritis Foundation. The following centers are involved: Erasmus Medical Center Rotterdam; Kennemer Gasthuis Haarlem; Leiden University Medical Center; Maastricht University Medical Center; Martini Hospital Groningen/Allied Health Care Center for Rheumatology and Rehabilitation Groningen; Medical Spectrum Twente Enschede/Ziekenhuisgroep Twente Almelo; Reade Center for Rehabilitation and Rheumatology; St Maartens‐kliniek Nijmegen; University Medical Center Utrecht; and Wilhelmina Hospital Assen. Mr. Salis is recipient of an Australian Government Research Training Program Scholarship. Dr. Sainsbury's work was supported by the National Health and Medical Research Council of Australia (Senior Research fellowship 1135897).
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.25057&file=acr25057‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Hunter DJ, Bierma‐Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 2. Fu M, Zhou H, Li Y, et al. Global, regional, and national burdens of hip osteoarthritis from 1990 to 2019: estimates from the 2019 Global Burden of Disease Study. Arthritis Res Ther 2022;24:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Royal Australian College of General Practitioners (RACGP) . Guideline for the management of knee and hip osteoarthritis. 2nd ed. 2018. URL: https://www.racgp.org.au/download/Documents/Guidelines/Musculoskeletal/guideline-for-the-management-of-knee-and-hip-oa-2nd-edition.pdf.
- 4. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020;72:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandes, L , Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non‐pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. [DOI] [PubMed] [Google Scholar]
- 6. Jevsevar DS, Brown GA, Jones DL, et al. The American Academy of Orthopaedic Surgeons evidence‐based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am 2013;95:1885–6. [DOI] [PubMed] [Google Scholar]
- 7. Brosseau L, Wells GA, Tugwell P, et al. Ottawa Panel evidence‐based clinical practice guidelines for the management of osteoarthritis in adults who are obese or overweight. Phys Ther 2011;91:843–61. [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence (NICE) . Osteoarthritis: care and management. NICE Clinical Guidelines, No. 177. London (UK): National Institute for Health and Care Excellence; 2020. [Google Scholar]
- 9. Murphy NJ, Eyles JP, Hunter DJ. Hip osteoarthritis: etiopathogenesis and implications for management. Adv Ther 2016;33:1921–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osteoarthritis Initiative . Osteoarthritis Initiative (OAI) study protocol. URL: https://nda.nih.gov/static/docs/StudyDesignProtocolAndAppendices.pdf.
- 11. Wesseling J, Boers M, Viergever MA, et al. Cohort profile: Cohort Hip and Cohort Knee (CHECK) study. Int J Epidemiol 2014;45:36–44. [DOI] [PubMed] [Google Scholar]
- 12. Croft P, Cooper C, Coggon D. Case definition of hip osteoarthritis in epidemiologic studies. J Rheumatol 1994;21:591–2. [PubMed] [Google Scholar]
- 13. Kellgren JH, Lawrence JS. Radiological assessment of osteo‐arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felson DT, Niu J, Guermazi A, et al. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis 2011;70:1884–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arden NK, Lane NE, Parimi N, et al. Defining incident radiographic hip osteoarthritis for epidemiologic studies in women. Arthritis Rheum 2009;60:1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lane NE, Nevitt MC, Hochberg MC, et al. Progression of radiographic hip osteoarthritis over eight years in a community sample of elderly white women. Arthritis Rheum 2004;50:1477–86. [DOI] [PubMed] [Google Scholar]
- 17. Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods 2004;7:127–50. [Google Scholar]
- 18. Zhang S, Su P, Liu S. Fusion of cognitive information: evaluation and evolution method of product image form. Comput Intell Neurosci 2021;2021:5524093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 20. Sorjonen K, Falkstedt D, Melin B, et al. The peril of adjusting for baseline when using change as a predictor. PsyArXiv doi: 10.31234/osf.io/6p5hj. 2019. E‐pub ahead of print. [DOI] [Google Scholar]
- 21. Wesseling J, Dekker J, van den Berg WB, et al. CHECK (Cohort Hip and Cohort Knee): similarities and differences with the Osteoarthritis Initiative. Ann Rheum Dis 2009;68:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heuts EA, de Jong LD, Hazebroek EJ, et al. The influence of bariatric surgery on hip and knee joint pain: a systematic review. Surg Obes Relat Dis 2021;17:1637–53. [DOI] [PubMed] [Google Scholar]
- 23. Paans N, van den Akker‐Scheek I, Dilling RG, et al. Effect of exercise and weight loss in people who have hip osteoarthritis and are overweight or obese: a prospective cohort study. Phys Ther 2013;93:137–46. [DOI] [PubMed] [Google Scholar]
- 24. Joseph GB, McCulloch CE, Nevitt MC, et al. Effects of weight change on knee and hip radiographic measurements and pain over 4 years: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2023;75:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin X, Gibson A, Gale J, et al. Does weight loss reduce the incidence of total knee and hip replacement for osteoarthritis? A prospective cohort study among older adults with overweight or obesity. Osteoarthritis Cartilage 2020;28 Suppl 1:S419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salis Z, Sainsbury A, Keen HI, et al. Weight loss is associated with reduced risk of knee and hip replacement: a survival analysis using Osteoarthritis Initiative data. Int J Obes (London) 2022;46:874–84. [DOI] [PubMed] [Google Scholar]
- 27. Salis Z, Sainsbury A. Association between change in body mass index and knee and hip replacements: a survival analysis of seven to ten years using multicohort data. Arthritis Care Res (Hoboken) 2023;75:1340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altman DG. Categorizing continuous variables. Wiley StatsRef: Statistics Reference Online. 2014. URL: 10.1002/9781118445112.stat04857. [DOI]
- 29. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. [DOI] [PubMed] [Google Scholar]
- 30. Christensen R, Bartels EM, Astrup A, et al. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta‐analysis. Ann Rheum Dis 2007;66:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol 2009;5:319–25. [DOI] [PubMed] [Google Scholar]
- 33. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 34. Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging 2008;12:487–91. [DOI] [PubMed] [Google Scholar]
- 35. Ensrud KE, Ewing SK, Stone KL, et al. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 2003;51:1740–7. [DOI] [PubMed] [Google Scholar]
- 36. Langlois JA, Visser M, Davidovic LS, et al. Hip fracture risk in older white men is associated with change in body weight from age 50 years to old age. Arch Intern Med 1998;158:990–6. [DOI] [PubMed] [Google Scholar]
- 37. Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc 2001;49:1309–18. [DOI] [PubMed] [Google Scholar]
- 38. Coker RH, Wolfe RR. Weight loss strategies in the elderly: a clinical conundrum. Obesity (Silver Spring) 2018;26:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Dijk GM, Dekker J, Veenhof C, et al. Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum 2006;55:779–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Supplementary Figure 1: Histogram of BMI change


