Figure 1.
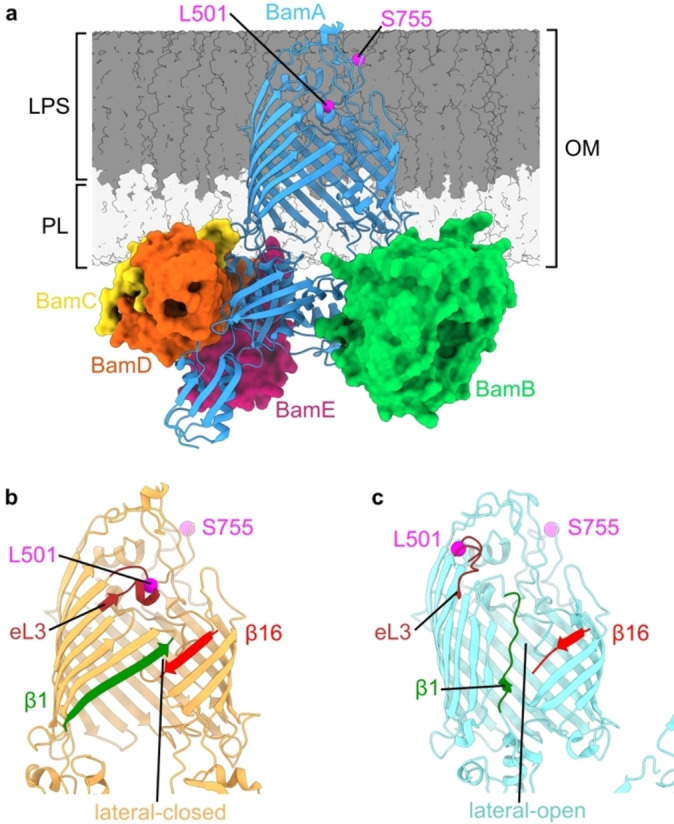
Conformational heterogeneity of the BAM complex. a) Structure of the full BamABCDE complex (PDB 5D0O). [5] Positions of L501 and S755, labelled in the L501R1‐S755R1 spin pair discussed in this manuscript, are indicated (magenta). Relative height of LPS and phospholipid (PL) portions of the OM are indicated. BAM adopts b) lateral‐closed (PDB 5D0O) [5] and c) lateral‐open (PDB 5LJO) [6] conformations, distinguished by closing or opening of a lateral‐gate between β‐strands β1 and β16 of the BamA β‐barrel. Opening also results in movement of the N‐terminal barrel face involving β‐strands β1‐β6, and the extracellular loops (eLs) between them. Extracellular loop 3 (eL3, brown) is highlighted as movement of this loop (which contains L501) leads to distance changes for the L501R1‐S755R1 pair. The BamBCDE subunits are hidden in (b) and (c) for clarity.
