Abstract
Background
The restoration of auricular cartilage is a major problem of otolaryngology. The low regenerative capacity of cartilage requires alternative approaches such as cell and tissue engineering. Stem cells are one of the ways to repair auricular cartilage damages. The aim of the investigation was the regeneration of an artificial defect of the auricular cartilage of rabbits after the intravenous injection of stem cells.
Materials and Methods
The study was carried out on rabbits. A narrow strip of auricular cartilage was surgically removed. A previously prepared suspension of homologous mesenchymal stem cells (5 million) in 0.5 ml physiological solution was injected into the vein of the opposite ear. Tissue samples from the site of the injury were collected after 1, 2, and 3 months. Histological examinations of the tissues were carried out after staining with fuchsin-eosin, azure II–eosin, and according to Weigert. In addition, the amount of interleukin-6 (IL-6) and the transforming growth factor β1 (TGF-β1) in the blood serum were determined.
Results
The main method of healing is the formation of a connective tissue scar. Yret, an increase of the number of fibroblasts and single islands of the newly formed auricular cartilage was found, which indicates the migration of the injected stem cells to the site of the damage and settling there. The intravenous injection of stem cells did not affect the secretion of pro-inflammatory IL-6, but significantly increased the amount of TGF-β1.
Conclusions
We assume that regenerative processes were stimulated. Nevertheless, they were aimed at quickly restoring the tissue integrity through the typical stages of scar formation. The restoration of cartilage integrity requires additional regulatory factors which will determine the chondrogenic differentiation of stem cells.
Keywords: auricular cartilage, stem cells, regeneration, inflammation, intercellular matrix
Abstract
Kontekstas
Ausies kremzliᶙ atkūrimas yra svarbi otolaringologijos problema. Kadangi kremzliᶙ regeneracijos pajėgumas yra nedidelis, būtini alternatyvūs sprendimai – pavyzdžiui, ląsteliᶙ ir audiniᶙ inžinerija. Kamieninės ląstelės yra vienas iš būdᶙ atkurti ausᶙ kremzlėms padarytą žalą. Šio tyrimo tikslas buvo dirbtinai suformuoto triušio ausies žalos defekto regeneracija po intraveninės kamieniniᶙ ląsteliᶙ injekcijos.
Medžiagos ir metodai
Šis tyrimas atliktas su triušiais. Chirurginiu būdu buvo pašalinta siaura triušio ausies kremzliᶙ juostelė. Iš anksto buvo paruošta homologizuota mezenchiminiᶙ kamieniniᶙ ląsteliᶙ (5 milijonai) suspensija su 0,5 ml fiziologinio tirpalo. Šios suspensijos buvo įšvirkšta į kitos ausies veną. Sužeidimo vietos audiniᶙ mėginiai imti po 1, 2 ir 3 mėnesiᶙ. Surinktᶙ audiniᶙ histologinis tyrimas buvo atliktas ištepus juos su fuchsinu ir eozinu, azūro II-eozinu bei naudojant Weigerto metodiką. Be to, krau jo serume buvo nustatytas interleukino-6 (IL-6) ir transformuojančio augimo faktoriaus β1 (TGF-β1) kiekis.
Rezultatai
Pagrindinis gijimo metodas yra jungiamojo audinio rando susidarymas. Buvo nustatytas fibroblastᶙ skaičiaus padidėjimas. Taip pat nustatyta naujai susidariusiᶙ ausies kremzliᶙ saleliᶙ. Tai rodo, kad įšvirkštos kamieninės ląstelės migruoja į sužeidimo vietą ir ten įsikuria. Intraveninė kamieniniᶙ ląsteliᶙ injekcija nepaveikė prouždegiminio IL-6 išskyrimo, tačiau reikšmingai padidino TGF-β1 kiekį.
Išvados
Darome prielaidą, kad buvo stimuliuojami regeneraciniai procesai. Tačiau jais siekta greitai atkurti audinio integralumą įprastiniais rando susidarymo etapais. Kremzliᶙ integralumui atkurti reikia papildomᶙ reguliuojamᶙjᶙ veiksniᶙ, kurie paskatintᶙ chondrogeninę kamieniniᶙ ląsteliᶙ diferenciaciją.
Raktažodžiai: ausies kremzlės, kamieninės ląstelės, regeneracija, uždegimas, tarpląstelinė matrica
Introduction
Auricular cartilage reconstruction is one of the main challenges in modern otolaryngology. The regenerative potential of cartilage tissue is low due to the small population of stem cells and differentiated chondrocytes. Tissue engineering is an innovative approach for cartilage reconstruction. The traditional approach involves surgical transplantation of autologous costal cartilage [1]. The main disadvantages of this method are possible pathological processes at the material collection site and insufficient aesthetic results. Polymeric materials based on biocompatible matrices provide an alternative for cartilage reconstruction. Natural [2-6], synthetic [7, 8], or hybrid [9] polymers create a scaffold for the cells. Biocompatible polymer materials have limited use due to issues with biocompatibility, biodegradation, and resorption in the body. Minor damage or fractures of the auricular cartilage do not require the use of a scaffold. These injuries can be repaired using cell engineering techniques. Cartilage defects can be repaired through the injection of stem cells cultivated in vitro or by stimulating resident perichondral stem cells.
Controlling the proliferative potential and differentiation of stem cells is one of the most challenging aspects of their use in clinical practice. In some cases, they may undergo oncological transformation. Abnormal differentiation of stem cells can occur even with slight changes in the microenvironment [10]. Perichondrial stem cells are employed to repair defects in elastic cartilage [11, 12]. Basic fibroblast growth factor (bFGF) initiates proliferative processes in vivo [13, 14]. Changes persist for 3 months after bFGF injection, and newly formed cartilage appears after 2 weeks. Antibodies to bFGF offer a means of controlling regeneration, but morphological changes become irreversible after 3 months. Other studies demonstrate the potential of stem cells to regenerate elastic cartilage [15]. Mesenchymal stem cells (MSCs) have been effective in regenerating auricular cartilage in rabbits. These stem cells originated from adipose tissue, bone marrow, and the ear. Local injection of a suspension of these cells contributed to the restoration of auricular cartilage [16]. Similar results have been obtained in other studies with near-perichondrial injection of adipose-derived MSCs [17]. An advantage of this method is the absence of immune reactions to the implant or reconstructive materials, as well as the potential for complete regeneration of cartilage defects. Moreover, there is no need for additional invasive procedures. The cells can be injected directly into the defect site or intravascularly with minimal damage to other tissues. Consequently, minimally invasive cartilage reconstruction is possible. Regeneration of damaged tissue also requires a response from the immune system, and proinflammatory cytokines can usually be detected in the area of damage [18].
Systemic injection of MSCs can have an immunomodulatory effect. A decrease in the inflammatory response to lipopolysaccharides has been reported [19]. The plasticity of MSCs allows for the initiation of both pro- and anti-inflammatory phenotypes, which underlies the stimulation of regeneration in damaged tissues [20].
Therefore, the injection of MSCs into the bloodstream potentially reduces inflammatory reactions at the site of damaged tissues and accelerates regenerative processes. Biologically active compounds secreted by MSCs also induce and expedite regeneration. Consequently, it is important to investigate the possibility of intravenous MSCs injection for the restoration of auricular cartilage.
The aim of this study was to regenerate the artificially defected rabbit auricular cartilage after intravenous injection of stem cells.
Materials and methods
Stem cells
The umbilical cord was surgically removed from an adult female rabbit under local anesthesia (Lidocaine, 2%, Darnitsa, Kyiv, Ukraine). The placenta, umbilical cord, and remnants of the amniotic membrane were mechanically separated and then placed in a physiological solution.
The umbilical cord (UC) was mechanically separated from the placenta and amnion. Subsequently, the UC was washed in phosphate buffer (PBS) and submerged in an antibiotic solution containing benzylpenicillin (20 units/ml, Arterium, Kyiv, Ukraine) and streptomycin (20 μg/ml, Arterium). Afterward, it was divided into fragments of 1x1 mm in size, placed in a solution of enzymes (1:1 (v/v) mixture of 0.2% collagenase and 0.1% trypsin), and incubated at 37°C for 1–2 hours until the tissues were visibly degraded to the maximum extent. Following this, the solution was inactivated using fetal bovine serum (FBS) and centrifuged at 1500 rpm for 10 minutes. The resulting pellet was resuspended and placed in a culture flask (25 or 75 cm2, SPL, Pocheon-si, South Korea) with DMEM nutrient medium (Biowest, Nuaillé, France) supplemented with benzylpenicillin (2 units/ml, Arterium), streptomycin (2 μg/ml, Arterium), and 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, Massachusetts, USA). The cells were incubated at 37°C under standard CO2 incubator conditions.
Once they reached sufficient confluence, primary culture clones were passed using a 1:1 mixture of EDTA (0.02%) and trypsin (0.1%), counted in a Goryaev’s chamber, and seeded at a density of 1x106 cells per vial (126 cm2). The same procedure was repeated for passage 1. At each passage, the cultures were observed using light and interference microscopy, employing a Leica DMIL inverted microscope (Wetzlar, Germany). At passage 1, the cells were assessed for their differentiation capacity for osteogenic and chondrogenic differentiation abilities, as described in our previous works [21]. MSCs at passage 2 were used for injection.
MSCs characterization
MSCs at passage 2 were utilized for experimentation. Throughout the cultivation process, at each passage, the cells exhibited strong adhesion to plastic surfaces and displayed a fibroblast-like morphology.
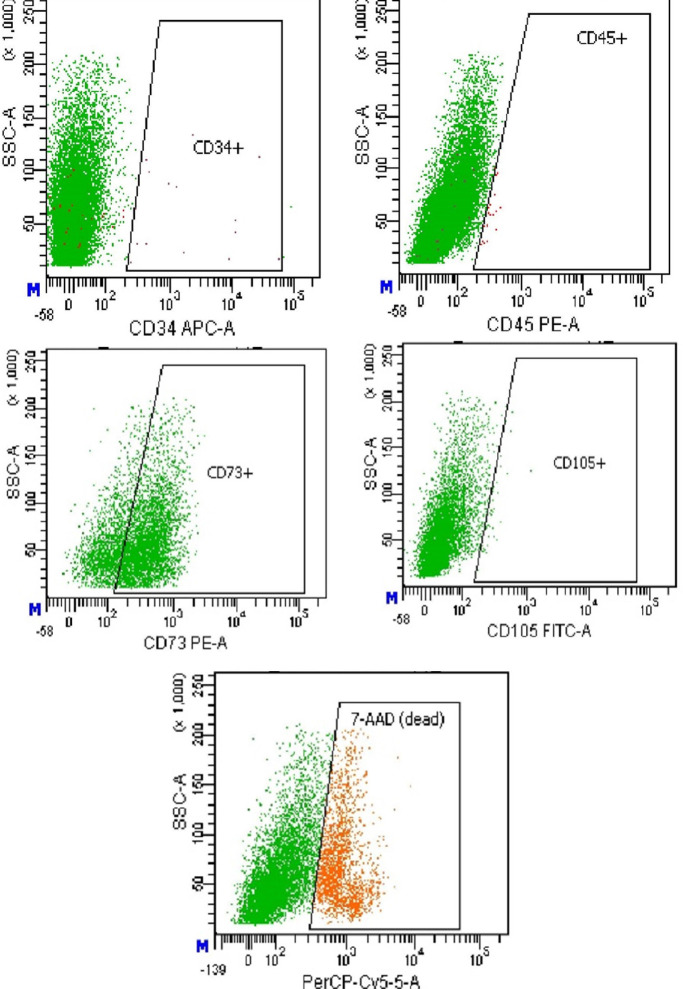
At passage 2, surface marker expression was assessed using flow cytometry on a FACSAria instrument (Beckton Dickinson Bioscience, New York, USA). Fluorescein isothiocyanate (FITC)-labeled anti-human CD105 and R-Phycoerythrin (PE)-labeled CD73 (US Biological) were employed, alongside anti-mouse CD34 (allophycocyanin (APC)-labeled) and CD45 (PE-labeled) (Beckton Dickinson), following the manufacturer’s instructions. The population was found to be negative for CD34 and CD45, while positive for CD73 (>70%) (Figure 1).
Figure 1.
Surface antigen expression by mesenchymal stem cells (MSCs) at passage 2: A – CD34, B – CD45, C – CD73, D – CD105. The number of living cells was about 75% of the total population (E); (flow cytometry by FACSAria).
Animals
Thirty rabbits of the ‘Gray Giant’ breed were selected for the study. The animals were divided into two groups: 12 in the control group and 18 in the experimental group. At the beginning of the investigation, the weight of the rabbits ranged within 1.5 kg.
The study adhered to the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and the Law of Ukraine “About the Protection of Animals from Cruel Treatment.” (Ethics Committee protocol #297, extract from the protocol dated 14 June 2023). The rabbits were individually kept in boxes under standard vivarium conditions at the State Institution “O.S. Kolomiychenko Institute of Otolaryngology of the National Academy of Medical Sciences of Ukraine.”
For anesthesia, xylazine hydrochloride (Xyla, 2%, Interchemie, Netherlands) was used at a dosage of 0.15 mg/kg. The ‘rabbit pinna punch-hole model’ was chosen as the basis for simulating auricular cartilage damage [22]. The area of the outer ear near the head was prepared by removing hair. An incision was made in the skin and cartilage, and a 10x1 mm fragment of auricular cartilage was excised. Afterward, the blood was removed with a sterile swab, and the wound was sutured. In the other ear, 5 million homologous MSCs (0.5 ml in saline) were injected into the lateral vein. The cell count was determined using a Goryaev chamber. The maximum number of cells used was that which did not induce changes in cytokines or trigger a significant immune system response. Control animals received a cell-free medium.
Auricular cartilage fragments with the damaged area were excised 1, 2, and 3 months after surgery, with the rabbits under general anesthesia. Additionally, blood samples were collected from the lateral ear vein for immunological studies.
Histological investigations
Histological sections of 15 µm thickness were prepared using the Microtome Cryostat HM525 (Microm, Thermo Scientific, Waltham, Massachusetts, USA) at -18 °C. Tissue visualization was achieved through hematoxylin-eosin staining [23]. Differential staining for collagen and elastin was performed following the van Gieson and Weigert methods [24-26]. The relative amount of basic and acidic components of the intercellular matrix was assessed after staining with azure-eosin [27], where acidic glycosaminoglycans appeared blue, and the basic tissue components exhibited various shades of red. Histological analysis was carried out using an Olympus BX53 microscope (Olympus, Shinjuku, Tokyo, Japan) equipped with an Olympus DP72 camera. Primary image processing was carried out using LabSens software.
Estimation of elastin mesh
Auricular cartilage defects were stained following the Weigert method [26]. The relative density of fibers in the tissue was quantified using ImageJ software. Comparisons were made with the connective tissue of the skin from the same histological preparation. The data were expressed in grayscale units ranging from 0 to 255, where 0 represents black and 255 represents white. An increase in the elastin network density corresponded to a decrease in grayscale values. Measurements were repeated 10 times. The results were presented as medians and quartiles.
Basophilic and oxyphilic tissue elements
Basophilic and oxyphilic tissue elements were visualized through staining with azure II-eosin. Basophilic tissue components appeared blue, while oxyphilic elements exhibited various shades of red. Image processing was performed using ImageJ software, analyzing the number of blue and red pixels in the image area. Diagrams illustrating the relative amount of oxyphilic and basophilic tissue components were constructed. These were then compared to similar graphs for the connective tissue of the skin in the same histological preparation. The ratio of oxyphilic to basophilic elements was calculated for the skin’s connective tissue (control), native auricular cartilage, and the tissues that filled the cartilage defect. Measurements were repeated 10 times. The results were presented as medians and quartiles.
Immunological studies
The enzyme immunoassay analysis (IFA) was used to determine interleukin-6 (IL-6) and transforming growth factor β1 (TGF-β1) in blood serum. RabbitIL-6 (Interleukin 6) ELISAKit and RabbitTGF-β1 (Transforming Growth Factor Beta 1) ELISAKit (Elabscience Biotechnology Inc., USA) were used. Blood was collected from the lateral ear vein of rabbits during surgery and 1, 2, 3 months after it. The blood was centrifuged at 1500 rpm. Corresponding antibodies were added to an aliquot of serum and the following manipulations were carried out according to the manufacturer’s recommendations. The optical density was measured on a Stat Fax (USA) tablet spectrophotometer. Statistical data processing was performed with the ANOVA Scheffé test (M±SD, p<0.05).
Results
Histological investigations
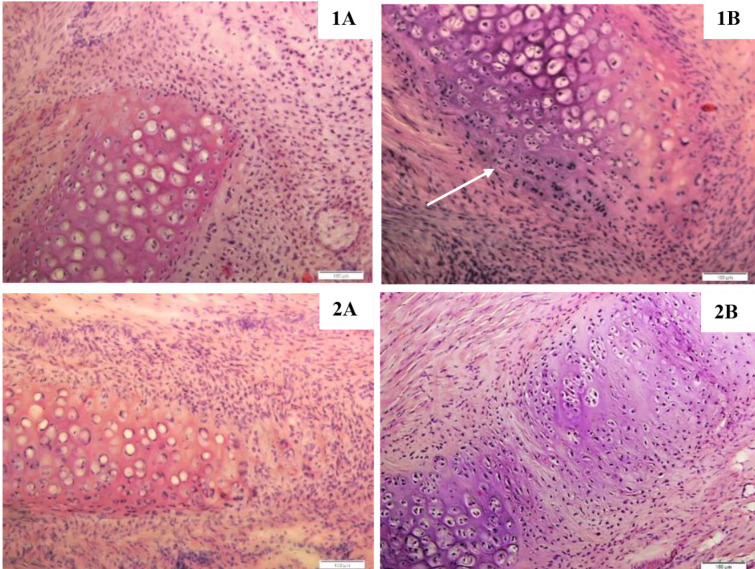
1 month after intravenous injection of MSCs, an increase in the number of fibroblasts around damaged cartilage in experimental rabbits was found (Figure 2, 1B). Areas of newly formed cartilage were observed, but they were separate fragments and did not form the necessary continuous structure (Figure 2, 1B; shown by arrow). The edge of the maternal cartilage was rounded, possibly due to inflammatory processes after surgery and/or the influence of stem cells.
Figure 2.
Morphological characteristics of artificially damaged rabbit auricular cartilage after one (1) and three months (2) after surgery. A – without MSCs injection, B – intravenous MSCs injection (staining by hematoxylin-eosin, magnification x100).
In control animals, a significant number of fibroblasts was found around the damaged cartilage (Figure 2, 1A). The edge of the cartilage was also rounded, but there were no zones of newly formed cartilage. Two and three months after the operation, the detected changes were preserved for control and experimental rabbits. Densification of the connective tissue, an increase in the number of fibers and fibroblasts at the site of damage were revealed (Figure 2, 2A, 2B). In none of the animals was the integrity of the auricular cartilage completely restored. Several specimens had satisfactory results after three months, but scar tissue was present (Figure 2, 2B). Thus, intravenous injection of stem cells has a limited effect on the repair of auricular cartilage.
Estimation of elastin mesh
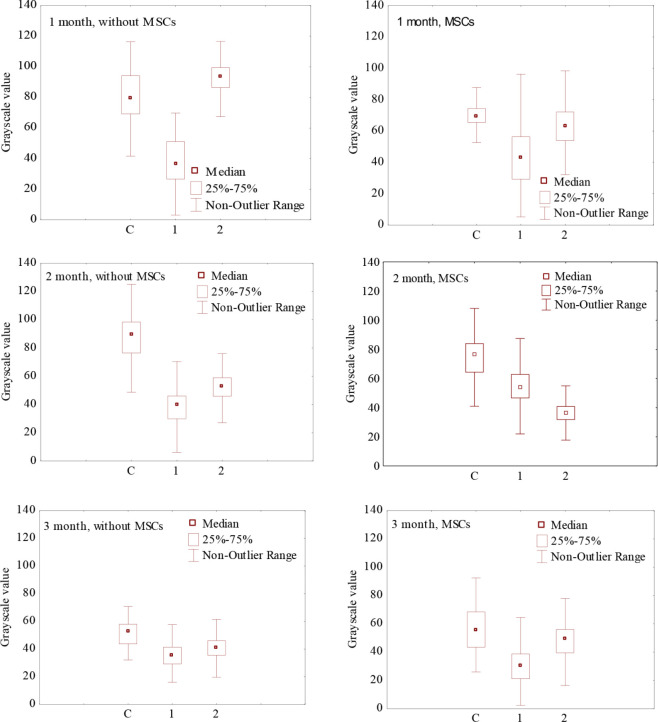
Analysis of the results was carried out on the same histological section due to slight differences in the intensity of staining of different samples. The relative density of the elastin mesh is presented in Figure 3.
Figure 3.
The density of the elastin mesh of the connective tissue of the skin (C), auricular cartilage (1) and tissue at the point of cartilage damage (2) for 1, 2 and 3 months after surgery (tissue staining by Weigert, grayscale value, medians and quartiles).
No significant differences were found between the newly formed tissues and the connective tissue of the skin. The results indicate the formation of a scar, which confirms the data of the general histological analysis. Nevertheless, an increase in the density of the elastin mesh was found in some areas of the auricular cartilage defect after MSCs injection. These changes were detected after 2 months (Figure 3). Such changes were found in few animals only, so it is impossible to reliably establish the influence of MSCs. Further research is needed to confirm this.
Basophilic and oxyphilic tissue elements
Staining with azure II-eosin differentiate of acidic and basic components of the intercellular matrix. Ear cartilage had many basophilic elements and almost no oxyphilic ones (Table 1).
Table 1.
The relative amount of basophilic and oxyphilic tissue elements of rabbit ear 1 month after surgery (median)
| Variant | Tissue | Grayvalue units | Ratio R/B | |
|---|---|---|---|---|
| Basophylic elements (blue, B) | Oxyphylic elements (red, R) | |||
| Without MSCs | Auricular cartilage | 57 | 15 | 0.26 |
| Damaged cartilage | 36 | 58 | 1.6 | |
| Skin connective tissue | 38 | 98 | 2.6 | |
| MSCs | Auricular cartilage | 35 | 4 | 0.11 |
| Damaged cartilage | 34 | 26 | 0.76 | |
| Skin connective tissue | 42 | 70 | 1.7 | |
1 month after MSCs injection, a high oxyphilia of the tissue filling the cartilage defect was detected. The ratio of oxyphilic and basophilic tissue components was within 0.7. This indicator was 2 times lower than the similar one calculated for the control group of rabbits (Table 1). The relationship was persisted after 2 months (Table 2). A lot of oxyphilic elements was present in the newly formed tissue.
Table 2.
The relative amount of basophilic and oxyphilic tissue elements of rabbit ear 2 months after surgery (median)
| Variant | Tissue | Grayvalue units | Ratio R/B | |
|---|---|---|---|---|
| Basophylic elements (blue, B) | Oxyphylic elements (red, R) | |||
| Without MSCs | Auricular cartilage | 38 | 8 | 0.21 |
| Damaged cartilage | 25 | 35 | 1.4 | |
| Skin connective tissue | 31 | 84 | 2.7 | |
| MSCs | Auricular cartilage | 25 | 4 | 0.16 |
| Damaged cartilage | 42 | 29 | 0.69 | |
| Skin connective tissue | 46 | 76 | 1.7 | |
There were no changes in experimental animals after three months (Table 3). The ratio of basophilic and oxyphilic elements 4:3 was preserved for experimental rabbits. The results indicate an early determination of the type of regenerated tissue and preservation of its properties.
Table 3.
The relative amount of basophilic and oxyphilic tissue elements of rabbit ear 3 months after surgery (median)
| Variant | Tissue | Grayvalue units | Ratio R/B | |
|---|---|---|---|---|
| Basophylic elements (blue, B) | Oxyphylic elements (red, R) | |||
| Without MSCs | Auricular cartilage | 34 | 4 | 0.12 |
| Damaged cartilage | 29 | 37 | 1.27 | |
| Skin connective tissue | 27 | 53 | 1.96 | |
| MSCs | Auricular cartilage | 28 | 3 | 0.11 |
| Damaged cartilage | 38 | 31 | 0.82 | |
| Skin connective tissue | 43 | 95 | 2.21 | |
Thus, intravenous injection of stem cells partially contributes to the initiation of regeneration processes of auricular cartilage damaged. The mechanisms underlying the process do not ensure the formation of full-fledged cartilage within 3 months after the start of treatment.
Immunological studies
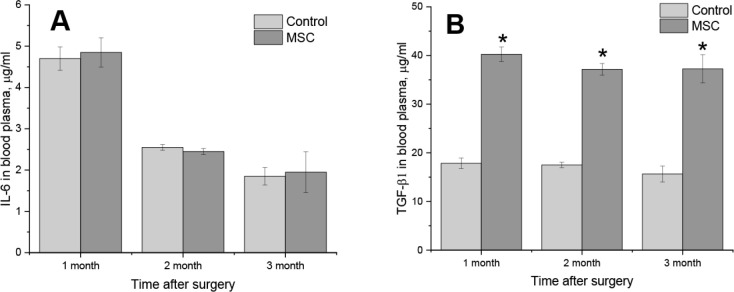
Increased concentrations of IL-6 in blood were detected for control and experimental rabbits after 1 month (Figure 4A).
Figure 4.
The concentration of IL-6 (A) and TGF-β1 (B) in the blood plasma of rabbits after 1, 2 and 3 months after the artificial auricular cartilage damage and the injection of stem cells. Control – animals without stem cells injection, MSC – intravenous injection of 5•106 of homologous mesenchymal stem cells in saline (M±SD, *p<0.05).
Intravenous injection of stem cells did not affect the amount of this cytokine. We assume that the main pro-inflammatory effect was caused by mechanical damage of the auricular cartilage. An almost two-time decrease of IL-6 concentration after 2 months was registered (Figure 4A). The identified changes indicate the termination of inflammatory processes.
A two-fold increase of TGF-β1 concentration in the blood of rabbits was detected after intravenous injection of stem cells compared to control animals (Figure 4b). These effects were remained for 3 months. Thus, intravenous injection of stem cells did not affect the secretion of pro-inflammatory IL-6, but significantly increased the amount of TGF-β1. Combined with histological studies, we assume that regenerative processes were stimulated.
Discussion
Cartilage regeneration stands as one of the central challenges in modern otolaryngology. The primary obstacle is the lack of cells which are already highly specialized. Hence, external sources of stem cells are often used. The cell type is recognized to be crucial for successful cartilage repair [28-30]. MSCs are particularly well-suited for cartilage regeneration due to their notable proliferative potential and the minimally invasive methods used for their extraction [31]. Various sources of stem cells are applied for cartilage regeneration, with chondrocytes obtained from the perichondrium of auricular cartilage being the most commonly used [32]. Adipose-derived and bone marrow-derived MSCs have also demonstrated positive effects on cartilage regeneration [16]. However, there remains a challenge of improper differentiation and the formation of cartilage ossification islands [33-35]. The injection of stem cells into the bloodstream leads to their dispersal throughout the body, settling in areas of tissue damage. The direction of their differentiation is determined by the local microenvironment and biologically active substances present. Interestingly, histological studies did not reveal significant differences in tissue regeneration following intravenous stem cell injection. We hypothesize that the enhancement of the effect may necessitate the presence of locally acting biologically active compounds, such as growth factors or cell differentiation regulators.
Auricular cartilage stands out due to its high content of elastin fibers, making it unique among other cartilage subtypes [36]. The surrounding tissues exhibit less organization and have a lower elastin content. We determined the relative density of the elastin mesh in native auricular cartilage, tissues in the damaged area, and the connective tissue of the skin through staining with lithium carmine. The density of the elastic fiber mesh at the site of artificial damage to the ear cartilage corresponds to that of connective tissue in a scar. However, we also identified tissue fragments in close proximity to the native cartilage, and we believe that the microenvironment contributed to this phenomenon.
Ear cartilage is known to contain a significant amount of glycosaminoglycans. These macromolecules consist of protein and repeating disaccharide residues, giving them a negative charge due to the abundance of sulfate groups [37]. We observed an increase in the acidic component of the tissue following the injection of MSCs. Our hypothesis is that stem cells, in conjunction with blood flow, reach the damaged auricular cartilage and can settle there. Their further differentiation is likely influenced by the local microenvironment and growth factors. In the immediate vicinity of the cartilage, differentiation into chondrocytes occurs, whereas at a distance, they tend to form a population of fibroblasts with varying degrees of differentiation.
Mechanical tissue damage is usually accompanied by inflammation [38]. IL-6, a pro-inflammatory cytokine, plays several key roles, including the modulation of interactions between T- and B-lymphocytes, the enhancement of T-killer cell activity, and the stimulation of granulocytic hematopoiesis [39]. During acute inflammatory processes, the levels of IL-6 increase rapidly and decline relatively quickly. In contrast, chronic inflammation is characterized by a sustained elevation of this cytokine in the blood over an extended period. TGF-family proteins are known to regulate the activity of CD44+ T-cells in the immune system and play a central role in the remodeling of the intercellular matrix [40, 41].
Conclusions
The intravenous injection of stem cells did not allow obtaining the stable regenerative processes of cartilage tissue and the restoration of the intercellular matrix to its original state. The main method of healing is the formation of a connective tissue scar, but an increase of fibroblasts number and single islands of the newly formed auricular cartilage were found, which indicates the migration of the injected stem cells to the site of damage and settling there. The microenvironment certainly plays a decisive role in their differentiation. Intravenous injection of stem cells did not affect the secretion of pro-inflammatory IL-6, but significantly increased the amount of TGF-β1. Combined with histological studies, we assume that regenerative processes were stimulated. Nevertheless, they were aimed at quickly restoring tissue integrity through the typical stages of scar formation. Restoration of cartilage integrity requires additional regulatory factors that will determine the chondrogenic differentiation of stem cells.
References
- 1.Bichara DA, O’Sullivan NA, Pomerantseva I, et al. The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev. 2012;18(1):51–61. doi: 10.1089/ten.TEB.2011.0326. [DOI] [PubMed] [Google Scholar]
- 2.de Chalain T, Phillips JH, Hinek A. Bioengineering of elastic cartilage with aggregated porcine and human auricular chondrocytes and hydrogels containing alginate, collagen, and kappa-elastin. J Biomed Mater Res. 1999;44(3):280–288. doi: 10.1002/(sici)1097-4636(19990305)44:3<280::aid-jbm6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.García-López J, Garciadiego-Cázares D, Melgarejo-Ramírez Y, et al. Chondrocyte differentiation for auricular cartilage reconstruction using a chitosan based hydrogel. Histol Histopathol. 2015;30(12):1477–1485. doi: 10.14670/HH-11-642. [DOI] [PubMed] [Google Scholar]
- 4.García-Martínez L, Campos F, Godoy-Guzmán C, et al. Encapsulation of human elastic cartilage-derived chondrocytes in nanostructured fibrin-agarose hydrogels. Histochem Cell Biol. 2017;147(1):83–95. doi: 10.1007/s00418-016-1485-9. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Yu F, Zheng L, et al. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J Orthop Translat. 2018;17:26–41. doi: 10.1016/j.jot.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4):42001. doi: 10.1088/1758-5090/ab331e. [DOI] [PubMed] [Google Scholar]
- 7.Stephan S, Reinisch J. Auricular Reconstruction Using Porous Polyethylene Implant Technique. Facial Plast Surg Clin North Am. 2018;26(1):69–85. doi: 10.1016/j.fsc.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Huang K, Zhu J, et al. A novel elastic and controlled-release poly(ether-ester-urethane)urea scaffold for cartilage regeneration. J Mater Chem B. 2020;18(8):4106–4121. doi: 10.1039/c9tb02754h. [DOI] [PubMed] [Google Scholar]
- 9.Chung JHY, Kade JC, Jeiranikhameneh A, et al. 3D hybrid printing platform for auricular cartilage reconstruction. Biomed Phys Eng Express. 2020;6(3):35003. doi: 10.1088/2057-1976/ab54a7. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi A, Ahmadi H, Pourfraidon Ghasrodashti Z, et al. Therapeutic effects of stem cells in different body systems, a novel method that is yet to gain trust: A comprehensive review. Bosn J Basic Med Sci. 2021;21(6):672–701. doi: 10.17305/bjbms.2021.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Takebe T, Inui M, et al. Reconstruction of human elastic cartilage by a CD44+ CD90+ stem cell in the ear perichondrium. Proc Natl Acad Sci USA. 2011;108(35):14479–14484. doi: 10.1073/pnas.1109767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagimoto S, Takebe T, Kobayashi S, et al. Autotransplantation of Monkey Ear Perichondrium-Derived Progenitor Cells for Cartilage Reconstruction. Cell Transplant. 2016;25(5):951–962. doi: 10.3727/096368916X690917. [DOI] [PubMed] [Google Scholar]
- 13.Miyanaga T, Ueda Y, Miyanaga A, Yagishita M, Hama N. Angiogenesis after administration of basic fibroblast growth factor induces proliferation and differentiation of mesenchymal stem cells in elastic perichondrium in an in vivo model: mini review of three sequential republication-abridged reports. Cell Mol Biol Lett. 2018;23:49. doi: 10.1186/s11658-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura G, Ebina K, Hirao M, et al. Promoting Effect of Basic Fibroblast Growth Factor in Synovial Mesenchymal Stem Cell-Based Cartilage Regeneration. Int J Mol Sci. 2020;22(1):300. doi: 10.3390/ijms22010300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein JL, Cohen BP, Lin A, Harper A, Bonassar LJ, Spector JA. Tissue Engineering Auricular Cartilage Using Late Passage Human Auricular Chondrocytes. Ann Plast Surg. 2018;80(4) Suppl 4:S168–S173. doi: 10.1097/SAP.0000000000001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan TA, Maher MA, El Karmoty AF, et al. Auricular cartilage regeneration using different types of mesenchymal stem cells in rabbits. Biol Res. 2022;55(1):40. doi: 10.1186/s40659-022-00408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh SJ, Park HY, Choi KU, et al. Auricular Cartilage Regeneration with Adipose-Derived Stem Cells in Rabbits. Mediators Inflamm. 2018;2018:4267158. doi: 10.1155/2018/4267158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, et al. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22(21):2825–2835. doi: 10.1089/scd.2013.0193. [DOI] [PubMed] [Google Scholar]
- 20.Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett. 2015;168(2):140–146. doi: 10.1016/j.imlet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Maslova OO, Shpilova SP, Shuvalova NS, Deryabina OG, Kordium VA. Spontaneous formation of spheroids in human umbilical cord matrix derived cells culture. Studia Biologica. 2012;6(2):79–86. doi: 10.30970/sbi.0602.212. [DOI] [Google Scholar]
- 22.ten Koppel PG, van Osch GJ, Verwoerd CD, Verwoerd-Verhoef HL. A new in vivo model for testing cartilage grafts and biomaterials: the ‘rabbit pinna punch-hole’ model. Biomaterials. 2001;22(11):1407–1414. doi: 10.1016/s0142-9612(00)00298-2. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(Suppl 3):S113–S116. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Puchtler H, Sweat F. Histochemical specifity of staining methods for connective tissue fibers: resorcin-fuchsin and van gieson’s picro-fuchsin. Z Zellforch Microsk Anat Histochem. 1964;79:24–34. doi: 10.1007/BF00304175. [DOI] [PubMed] [Google Scholar]
- 25.Prentø P. Van Gieson’s picrofuchsin. The staining mechanisms for collagen and cytoplasm, and an examination of the dye diffusion rate model of differential staining. Histochemistry. 1993;99(2):163–174. doi: 10.1007/BF00571877. [DOI] [PubMed] [Google Scholar]
- 26.Suvarna K, Layton C, Bancroft J. Bancroft’s Theory and Practice of Histological Techniques . Churchill Livingstone; 2019 doi: 10.1016/C2015-0-00143-5. [DOI] [Google Scholar]
- 27.Cross RF, Moorhead PD. An azure and eosin rapid staining technique. Can J Comp Med. 1969;33(4):317. [PMC free article] [PubMed] [Google Scholar]
- 28.Hou M, Bai B, Tian B, et al. Cartilage Regeneration Characteristics of Human and Goat Auricular Chondrocytes. Front Bioeng Biotechnol. 2021;9:766363. doi: 10.3389/fbioe.2021.766363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Zhu L, Liu Y, et al. Stable subcutaneous cartilage regeneration of bone marrow stromal cells directed by chondrocyte sheet. Acta Biomater. 2017;54:321–332. doi: 10.1016/j.actbio.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 30.3Yang Y, Lin H, Shen H, Wang B, Lei G, Tuan RS. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018;69:71–82. doi: 10.1016/j.actbio.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Yuan M, Guo QY, Lu SB, Peng J. Mesenchymal Stem Cells for Treating Articular Cartilage Defects and Osteoarthritis. Cell Transplant. 2015;24(9):1661–1678. doi: 10.3727/096368914X683485. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Duan L, Li Y, She Y, Zhu J, Zhou G, Jiang G, Yang Y. Nanofibrillar Decellularized Wharton’s Jelly Matrix for Segmental Tracheal Repair. Adv Funct Mater. 2020;30(14):1910067. doi: 10.1002/adfm.201910067. [DOI] [Google Scholar]
- 33.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.3Dickhut A, Pelttari K, Janicki P, Wagner W, Eckstein V, Egermann M, Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219(1):219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 35.Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med. 2009;266(4):390–405. doi: 10.1111/j.1365-2796.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 36.Bos EJ, Pluemeekers M, Helder M, et al. Structural and Mechanical Comparison of Human Ear, Alar, and Septal Cartilage. Plast Reconstr Surg Glob Open. 2018;6(1):e1610. doi: 10.1097/GOX.0000000000001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner W, Sakiyama-Elbert S, Zhang G, Yaszemski M. 4th ed. Academic Press; 2020. Biomaterials Science . [DOI] [Google Scholar]
- 38.Bortolotti P, Faure E, Kipnis E. Inflammasomes in Tissue Damages and Immune Disorders After Trauma. Front Immunol. 2018;9:1900. doi: 10.3389/fimmu.2018.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.xxYang YC, Zhang N, Van Crombruggen K, Hu GH, Hong SL, Bachert C. Transforming growth factor-beta1 in inflammatory airway disease: a key for understanding inflammation and remodeling. Allergy. 2012;67(10):1193–1202. doi: 10.1111/j.1398-9995.2012.02880.x. [DOI] [PubMed] [Google Scholar]