Fig. 7.
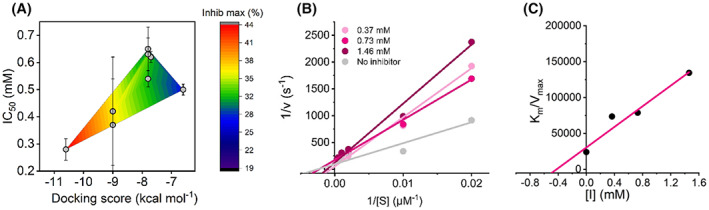
Additional kinetic investigation into the SCRA inhibition of MAO‐B turnover. (A) Comparison of in silico and in vitro data; the experimentally determined IC50 values of eight compounds are plotted against the computationally determined docking score. The overlaid heat map indicates the relationship of the maximum % inhibition with respect to the other parameters. (B) Kinetics study of the mechanism of MAO‐B inhibition by compound 8. A Lineweaver‐Burk plot for MAO‐B inhibition by 8 has been plotted where substrate concentrations of 50–3000 mm BZA were used in conjunction with three inhibitor concentrations. (C) Plot of K M/V max versus inhibitor concentration for the determination of the K i value of compound 8. Conditions, 24 μm MAO‐B, 50 mm HEPES, pH 7.5 + 0.5% Triton X‐100, All data were collected in triplicate and error bars indicate standard error.
