Fig. 1.
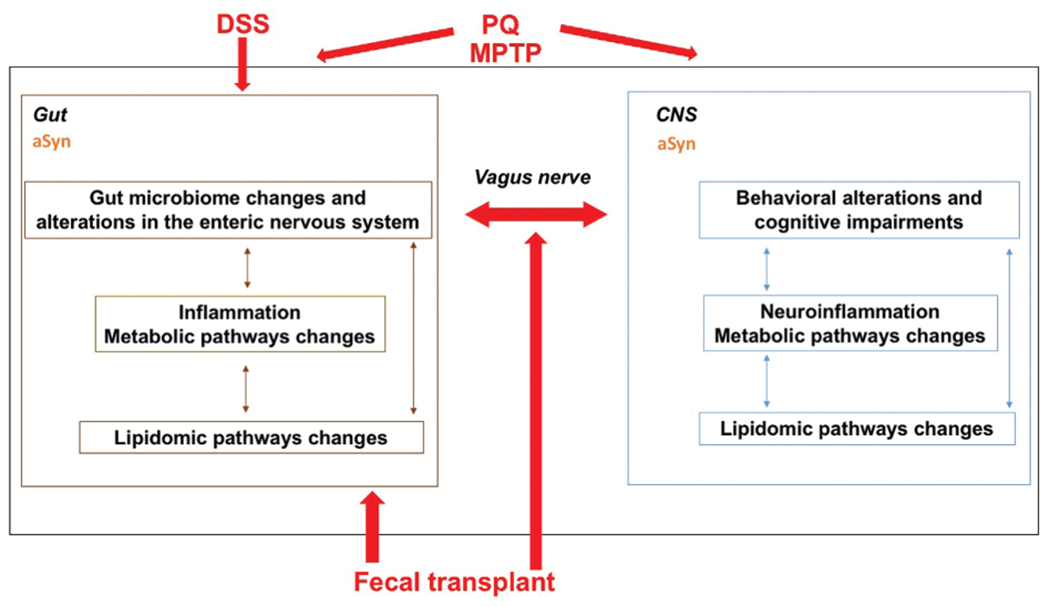
While the gut has historically not been considered a major contributor to PD, many PD patients have digestive symptoms years before they exhibit neurological symptoms, and composition of the gut microbiome of PD patients differs from that of healthy controls. It has been hypothesized that environmental toxins such as PQ or PD-related genetic mutations causing aggregation of aSyn, a major constituent of Lewy bodies and the pathological hallmark of PD, might be the initial trigger causing PD. Consistent with these human studies, gut microbiota regulate motor impairments and neuroinflammation in mice overexpressing aSyn. Moreover, flies mutant for parkin, important in PD pathogenesis and in gut enterocytes for the maintenance of microbial load homeostasis, are more sensitive to PQ and this sensitivity is reduced in germ-free flies. There is evidence that the vagus nerve is critical for mediating these gut–brain axis effects and for spreading aSyn pathology to the brain; vagotomy prevents the development of PD symptoms and constrains the appearance of misfolded aSyn to myenteric neurons following co-administration of subthreshold doses of PQ and lectins. Additionally, when transgenic mice displaying aSyn overexpression due to the A53T PD-associated mutation are chronically exposed to DSS, there is an invasion of macrophages into the lining of the gut wall, accumulation of aSyn in enteric neurons within the submucosal plexus, greater behavioral impairments, brain pathology, and inflammation in the gut and the brain. Consistent with these data, the effects of the neurotoxin MPTP on cognitive performance may, at least in part, be mediated by the gut microbiome. In the gut and the brain, gut microbiome changes might be mediated by inflammation involving alterations in metabolic pathways and lipidomic pathway changes. Fecal transplants of PD patients can induce PD phenotype in mice and fecal transplants of healthy people might be able to modulate PD phenotypes in PD patients. As the gut–brain axis involves bidirectional communication, PD pathology in the brain might also enhance PD pathology in the gut.
