Abstract
Rabies is a lethal viral disease transmitted through the bite of rabid animals. India has a high burden of rabies, contributing to a significant proportion of the global deaths. However, under-reporting of the disease is prevalent due to lack of laboratory confirmation. Laboratory diagnosis of rabies plays a crucial role in differentiating the disease from clinical mimics, initiation of appropriate care, implementing infection control measures and informing disease surveillance. This review provides an overview of the recent advancements in laboratory diagnosis of rabies, aimed at updating physicians involved in diagnosis and management of rabies cases in India.
Keywords: Ante-mortem diagnosis, human rabies, laboratory diagnosis, post-mortem diagnosis, rabies, rabies control, rabies surveillance
Rabies is a fatal viral encephalomyelitic disease primarily transmitted through the bites of rabid animals, especially dogs. Globally, it causes approximately 60,000 deaths annually, with India bearing the highest burden, accounting for one-third of all cases1. Rabies is endemic throughout India, except for the Andaman and Nicobar and Lakshadweep Islands. This zoonotic disease is caused by viruses of the Lyssavirus genus (Rhabdoviridae family and Mononegavirales order), with the rabies virus (RABV) being the predominant causative agent in India.
The lack of laboratory confirmation contributes to the under-reporting of rabies in India. However, recognizing the importance of accurate reporting, human rabies has been made a notifiable disease in the country2. Consequently, laboratory testing to confirm or rule out rabies diagnosis in suspected cases has gained increased significance.
To improve public health surveillance and effectively manage rabies cases, it is crucial to prioritize laboratory confirmation of the disease, which will enable accurate reporting, facilitate appropriate treatment and support targeted prevention and control measures. In this context, the current article presents a comprehensive review of literature, spanning the past two decades, highlighting the critical role of laboratory confirmation in the diagnosis of rabies. It specifically examines standard algorithms, recent advancements and the challenges encountered in laboratory diagnosis. Overall aim of this review is to provide insights to physicians involved in the diagnosis and management of rabies cases in India, enabling them to make informed decisions based on the latest developments in the field.
Methods
A comprehensive search on Google Scholar and the PubMed database was conducted for the literature published between January 1, 2003 and December 31, 2022. The search terms included ‘rabies in India’, ‘laboratory diagnosis of rabies’, ‘rabies surveillance’, ‘national rabies control programme’ and ‘new rabies diagnostic tests’. A variety of publications, including original research articles, systematic reviews, book chapters, standard manuals and international and national health policies and reports were reviewed. We also examined publications that evaluated new tests against the gold standard, even if these were not yet incorporated into the standard testing algorithms.
Need for laboratory confirmation
Clinical rabies and differential diagnoses: Rabies presents in two clinical forms: encephalitic (furious) and paralytic (dumb). Symptoms typically appear within 20-90 days after exposure; however, the incubation period can vary from a week to more than a year1. Non-specific prodromal symptoms include fever, paraesthesias, pain or pruritis near the site of exposure, malaise, sore throat, dysphagia, anxiety, irritability, insomnia and restlessness. The neurological phase is characterized by fluctuating consciousness, spasms, autonomic dysfunction and hydrophobia in encephalitic rabies1,3. Paralytic rabies is characterized by gradual onset of ascending flaccid paralysis. It typically starts in the bitten extremity and progressively spreads to involve all limbs, as well as the pharyngeal and respiratory muscles3,4. Hydrophobia is uncommon. Without intensive care, both forms eventually lead to coma and death within 1-2 weeks of symptom onset5.
The encephalitic form of rabies, characterized by classical clinical features and rapid progression in the presence of a history of rabid exposure, usually does not require laboratory confirmation. However, diagnosing rabies becomes challenging in affected individuals presenting with paralytic or atypical features, particularly when there is no history of exposure. While the encephalitic form is considered more prevalent, there has been an increase in reported cases with paralytic/atypical presentations in recent years from India6,7,8.
Paralytic rabies can be mimicked by Guillain–Barre syndrome, including acute inflammatory demyelinating polyradiculopathy and acute motor-sensory axonal neuropathy. Other differential diagnoses to consider include post-vaccinal encephalomyelitis, although rare due to the ban on nerve-tissue-derived rabies vaccines in India since 2005. Additional conditions that resemble rabies include anti-N-methyl-d-aspartate receptor encephalitis, infectious aetiologies such as tetanus, cerebral malaria, herpes simplex and other viral encephalitis, as well as rabies hysteria, psychiatric disorders, scorpion and snake envenomations, illicit drug use and organophosphate poisoning9,10,11,12. While clinical features, neuroimaging and routine laboratory tests can help differentiate these conditions from rabies, specific laboratory tests are required for definitive confirmation or exclusion of a rabies diagnosis.
Clinical management: Early laboratory confirmation of rabies plays a crucial role in avoiding unnecessary investigations and treatments, allowing for initiation of appropriate supportive and palliative care, with infection control measures. Furthermore, timely confirmation aids in effectively communicating the prognosis to family members, facilitating case closure and providing necessary grief counselling12,13.
Currently, there is no effective treatment for rabies, and the disease is considered virtually fatal once symptoms appear. Supportive care, including sedation, rehydration, analgesia and antipyretics, is provided to alleviate suffering. Fewer than 30 cases of survival from rabies are documented worldwide14; more than 15 of these cases have been reported from India, mostly in the last decade6,14,15,16,17,18. Several other cases have shown prolonged survival ranging from weeks to months7,8,19,20. It is possible that additional cases with similar outcomes in India have gone undiagnosed or unreported until recent years. This evolving clinical profile of rabies survival was previously unknown in India, except for a single reported case of partial recovery in 200221.
In the last decade, a protracted clinical course and longer survival in some rabies cases were witnessed which can be attributed to factors such as prior partial or complete rabies vaccination, increased awareness about rabies, improved access to medical care in India6,15,16,17,18,19,20,22,23, ante-mortem laboratory confirmation and effective intensive care and supportive treatment19. While most survivors experienced moderate-to-severe neurological sequelae, a few cases from India and elsewhere have shown almost complete recovery with minimal sequelae18,22,23,24. Such rare cases with favourable outcomes have renewed hope and sparked interest in exploring aggressive management and experimental protocols among physicians and family members of individuals affected by this devastating disease.
Rapid advancements in medicine provide reasons for optimism regarding improved outcomes in rabies in the near future. The availability of ante-mortem diagnostic facilities plays a crucial role in early laboratory confirmation of rabies and monitoring therapeutic response in cases where aggressive management is attempted19,22,25. Most documented rabies survivors with or without treatment exhibited an early as well as a robust immune response, diagnosed solely by the presence of neutralizing antibodies in cerebrospinal fluid (CSF) and/or serum, without detectable viral antigen or nucleic acid14,18,26. A study on laboratory-confirmed rabies cases in India found that individuals who tested positive only for neutralizing antibodies at initial diagnosis had a longer duration of survival compared to those who tested positive for viral nucleic acid, with or without the neutralizing antibodies7. Young individuals, previously vaccinated, presenting in the early phase of illness, with negative diagnostic tests for rabies viral antigen/RNA and positive tests for rabies neutralizing antibodies in CSF/serum, are considered favorable candidates for attempting aggressive management in critical-care units14. The availability of laboratory testing aids clinicians in making crucial decisions regarding supportive, palliative or aggressive management and helps resolve the moral and ethical dilemmas they may encounter in such cases.
In 2005, a teenager in the USA survived rabies after receiving experimental treatment involving therapeutic coma, anti-excitotoxic measures and antiviral strategies22. However, subsequent attempts to replicate this success in other cases, including some from India, have largely been unsuccessful25,27,28,29. The treatment protocol has undergone several revisions, and the current version emphasizes supportive critical care, with a focus on preventing fatal dysautonomia in the early stages while allowing the natural development of neutralizing antibodies to clear the infection11,30.
The recent WHO consultation emphasizes the need for carefully planned and validated studies conducted in an ethical manner to explore new management protocols, immunomodulation procedures and potential antiviral drugs for treatment of rabies1. In a country where the death toll from rabies remains alarmingly high, it is crucial for selected medical academic institutes in India with appropriate diagnostic, clinical and research capabilities to step up and embrace this challenge. These institutes are well positioned to contribute significantly to the development and implementation of innovative approaches to combat this deadly disease.
Infection control and public health: Human-to-human transmission of rabies is rare, typically occurring through infected tissue or organ transplants. Standard precautions, such as avoiding contact with rabies-infected biological material on mucosal surfaces and broken skin during procedures such as intubation and suctioning, significantly reduce the risk of transmission to healthcare staff. Close contacts of patients, including family members and healthcare workers, should receive post-exposure prophylaxis based on individual risk assessment1. Laboratory confirmation plays a crucial role in assessing the risk and guiding appropriate preventive measures for personnel involved in embalming, autopsy or disposal of cadavers.
Reported cases of rabies transmission through solid organ or tissue transplantation worldwide highlight the importance of screening donors, especially those with unexplained neurological illnesses and other risk factors, in countries like India, where rabies is endemic31,32,33. As the number of tissue and organ transplants increases, it becomes critical to implement screening protocols to prevent the inadvertent transmission of rabies to recipients.
Surveillance: The under-reporting of rabies cases posses a significant challenge in tackling this neglected zoonotic disease. To achieve the global goal of eliminating human deaths due to dog-mediated rabies by 203034, the National Rabies Control Programme (NRCP) in India has developed the National Action Plan for Dog Mediated Rabies Elimination (NAPRE)35. This plan emphasizes the importance of systematic disease surveillance and adopts a comprehensive ‘One Health’ approach to control rabies.
As part of the NAPRE, establishment of rabies diagnostic laboratories at the national, regional and State levels is planned to strengthen laboratory-based surveillance of human and animal rabies35. This initiative aims to enhance the reporting and monitoring of rabies cases, enabling a more accurate assessment of the disease burden.
A significant step towards rabies elimination in India is the recent declaration of human rabies as a notifiable disease2. This directive issued by the Government of India to all States emphasizes the importance of timely reporting and monitoring of human rabies cases, facilitating effective disease control measures.
Disease surveillance plays a crucial role in the NRCP by enabling estimation of disease burden, early detection and monitoring of outbreaks and evaluation of intervention effectiveness. Laboratory-based surveillance, in particular, is an essential component of this programme.
Laboratory testing is instrumental in confirming and characterizing rabies cases. It allows for the identification of the virus through techniques such as partial or whole-genome sequencing. This detailed characterization of the virus provides valuable insights into disease epidemiology, including identification of circulating variants, presence of non-RABV lyssaviruses, host species involved, geographical origins and sources of infection36,37,38.
The genomic diversity of RABV poses a challenge for PCR-based detection methods that rely on amplification of specific target sequences. In some cases, genetic variations within the target regions can prevent appropriate binding of primers and probes, leading to a failure in detection of the viral nucleic acids and producing a false-negative result39,40,41,42,43. Regular monitoring and genomic characterization of RABV strains can aid in development of effective PCR assays that can account for genetic diversity and provide accurate results.
RABV is a member of the lyssaviruses, a diverse group of viruses consisting of more than 16 genetically distinct viruses26. While RABV is the primary cause of human rabies deaths worldwide, all lyssaviruses have the potential to cause the disease. Presence of other lyssaviruses in India is not well defined. In addition to dogs being the main source of rabies transmission in most cases, the role of wildlife and bats in transmitting lyssaviruses to humans is significantly underreported in India, despite global reports of such incidents44. Recent detection of a novel lyssavirus called Gannoruwa bat lyssavirus in fruit bats in neighbouring Sri Lanka and a study conducted on bats in one of the north-eastern States of India suggest that lyssaviruses may be circulating within bat populations across the country45,46.
Genomic data serve as a powerful tool for surveillance, providing insights into the spread of rabies and guiding control efforts. Next-generation sequencing has made it easier to obtain complete lyssavirus genomes, enabling comprehensive analysis of viral transmission patterns47,48. Studying the phylogenetic landscape using genomic data helps quantify interstate and inter-region transmission, aiding the development of effective national strategies for rabies control and monitoring the impact of mass dog vaccination programmes. Moreover, enhanced surveillance for lyssavirus diversity will contribute to our understanding of the effectiveness of existing biologics used for the prevention and control of rabies.
In countries or regions that have witnessed a decline in human rabies cases and are actively working towards rabies elimination, ongoing laboratory-based surveillance becomes even more crucial. This is evident in the case of Goa, a popular global tourist destination in India, which has made significant progress towards canine rabies elimination, with no human deaths reported in the past four year49,50. Sustained laboratory-based surveillance plays a vital role in monitoring and verifying the achievement of this milestone, ensuring that the progress is maintained and potential outbreaks are promptly detected and addressed.
Similarly, for regions like the Indian islands of Andaman, Nicobar and Lakshadweep, continual laboratory-based surveillance is essential to ensure maintenance of their disease-free status51. Even in areas that have remained rabies-free for an extended period, the risk of reintroduction of the virus is ever-present. A prime example is Malaysia, which recently experienced an outbreak of canine rabies after 20 years without any reported cases52. This outbreak resulted in more than 15 human deaths, emphasizing the persistent threat of transboundary incursions and underlines the need for vigilant surveillance.
Laboratory diagnostic tests for rabies
Routine laboratory investigations are likely to be normal in the initial stage, except for mild pleocytosis and slightly elevated protein in CSF, and do not aid in diagnosis. Specific laboratory tests are required to confirm rabies and can be performed on clinical specimens obtained ante-mortem or post-mortem1,7,8,11,12,13,26,41,53,54,55,56,57,58,59,60,61,62,63,64,65,66 (Table).
Table.
Laboratory tests for diagnosis of human rabies
| Laboratory test | Clinical sample | Advantages | Disadvantages | Comments |
|---|---|---|---|---|
|
| ||||
| Detection of viral antigen | ||||
| FAT | Brain tissue | -Nearly 100% sensitive and specific - Gold standard for post-mortem laboratory confirmation of rabies | Fresh brain smears are ideal for best results; Formalin-fixed, stored, decomposed or autolyzed brain sample does not give reliable results | Short turnaround time (2-3 h) Requires good quality of sample, biosafety containment, expensive fluorescence microscope and technical expertise |
| Nuchal skin biopsy | Moderate sensitivity | -Time-consuming and cumbersome technique, several sections around the base of the hair follicles (with peripheral nerves) need to be examined - For the preparation of frozen sections, the cryostat is required (not available in all laboratories) | ||
| Corneal impressions | Simple technique | Less reliable (low sensitivity and specificity) Not recommended due to the risk of corneal injuries | ||
| dRIT | Brain tissue | -Nearly 100% sensitive and specific Can be used on frozen or glycerol-preserved brain tissues - Requires only light microscope, does not require expensive fluorescence microscope (ideal for field use) | -Recommended for post-mortem diagnosis of rabies in animals; not adequately validated for rabies diagnosis in humans -Complex technical assay requires multiple washing steps and training | Short turnaround time (2-3 h) Reagents not available commercially |
| RIDT | Brain tissue | Used as a point of sampling test in the field (surveillance) | Commercially available kits lack standardization and stringent quality control; varied sensitivity and specificity Useful for rapid post-mortem rabies diagnosis in animals, not adequately validated in human samples | Short turnaround time (10-20 min) Can be used in situations where laboratory diagnostic facilities are lacking |
| Detection of viral antibodies | ||||
|
| ||||
| RFFIT/FAVN Detect virus-neutralizing antibodies ELISA Detects antibodies against rabies glycoprotein | CSF | Presence of RVNA in CSF is diagnostic of rabies irrespective of vaccination status | Low sensitivity (due to short survival and late appearance of RVNA in rabies) | -Sensitivity increases with increased duration of survival RFFIT/FAVN: cell culture-based assay; labour intensive and long turnaround time (2-3 days) Poor sample quality affects results (cytotoxicity) |
| -Requires biosafety containment (for handling live virus), fluorescence microscope, strict adherence to quality control, skilled technical staff and analyst ELISA: Short turnaround time, inexpensive, no handling of live rabies virus (safe); when RFFIT/FAVN is not available, adequately validated ELISA can be used | ||||
| Serum | Presence of RVNA is diagnostic of rabies in unvaccinated individuals Can be used to estimate post-vaccination antibody levels; RVNA titres ≥0.5 IU/ml suggest adequate seroconversion | In previously vaccinated individuals, 4-fold rise of titres in paired sera has to be demonstrated to confirm the diagnosis | ||
| Detection of viral RNA (nucleic acid amplification technique) | ||||
|
| ||||
| Conventional and real-time RT-PCR | Saliva | Easy, non-invasive sample collection High sensitivity | Pooled/serial salivary secretions (3 samples collected at an interval of 3-6 h) have to be tested for increased sensitivity, due to intermittent shedding of virus | Short turnaround time (3-4 h) Sample integrity, proper storage and transport (cold chain) should be maintained Negative test result cannot rule out the diagnosis of rabies in samples other than brain tissue |
| Nuchal skin biopsy | High sensitivity | Invasive procedure (excision or punch biopsy) Full-thickness biopsy with adequate hair follicles for increased sensitivity | ||
| CSF | Sample commonly collected for all neurological investigations | Invasive procedure (lumbar puncture) Low sensitivity | ||
| Brain tissue | 100% sensitive and specific | Collection of post-mortem brain sample challenging | ||
| Virus isolation | ||||
|
| ||||
| RTCIT | CSF and saliva Brain tissue | Can cultivate and passage the virus (murine neuroblastoma cell lines) to obtain a large amount of virus for molecular (genomic) characterization | Low sensitivity Not suitable for routine diagnosis; can be done only in referral laboratories | Long turnaround time (2-4 days) Requires cell culture facility, biosafety containment and technical expertise |
| MIT | Brain tissue | Large amount of virus can be isolated from a single mouse brain (suckling, weaning or adult) for molecular (genomic) characterization | Not recommended for routine diagnosis; requires animal facility, specialized laboratory with biosafety containment and ethical approval | Very long turnaround time (up to 28 days) |
| Laboratory test | Clinical sample | Advantages | Disadvantages | Comments |
| Histological identification of Negri bodies | ||||
|
| ||||
| Seller’s technique | Brain tissue | Simple and rapid method | Negative test result cannot rule out rabies, hence not recommended for primary diagnosis; can be used as a complementary test | H and E staining on formalin-fixed tissues – time-consuming (5-7 days) |
| H and E staining | Brain tissue | Less biohazard (formalin-fixed, paraffin-embedded tissues) | ||
FAT, fluorescent antibody technique; dRIT, direct rapid immunohistochemical test; RIDT, rapid immunochromatographic diagnostic test; RFFIT, rapid fluorescent focus inhibition test; FAVN, fluorescent antibody virus neutralization test; ELISA, enzyme-linked immunosorbent assay; CSF, cerebrospinal fluid; RVNA: rabies virus-neutralizing antibodies; RT-PCR, reverse transcriptase–polymerase chain reaction; RTCIT, rabies tissue culture infection test; MIT, mouse inoculation test; H and E: Haematoxylin and Eosin
Laboratory diagnosis of rabies is possible only after the onset of the illness; no tests can diagnose it during the incubation period.
Diagnostic modalities: There are various techniques available for the laboratory diagnosis of rabies. These are broadly classified as follows:
Viral antigen detection: One of the earliest and most reliable techniques for post-mortem diagnosis of rabies is direct fluorescent antibody test (FAT). Even today, it remains the gold standard for confirming rabies in human and animal post-mortem samples1,53. It is based on the detection of the rabies nucleoprotein antigen in touch impression brain smears, using specific rabies antibodies labelled with fluorescein isothiocyanate, allowing for visualization of the antigen under fluorescence microscopy. An alternative method, the direct rapid immunohistochemical test (dRIT), utilizes a cocktail of biotinylated monoclonal antibodies and can be viewed under a light microscope. Unlike FAT, dRIT does not require the use of fluorescence microscopy, making it more accessible in laboratories with basic facilities for diagnostic testing55. FAT can be employed for antigen detection in samples such as corneal smears and nuchal skin biopsies. However, the sensitivity and specificity of FAT are relatively low in these samples. Furthermore, the use of corneal smears is discouraged due to the potential risk of corneal scarification1,12.
A rabies diagnosis can be confirmed by histopathologically detecting the distinctive eosinophilic, intracytoplasmic inclusions known as ‘Negri bodies’ in formalin-fixed brain tissues. A negative result cannot, however, conclusively rule out the diagnosis of rabies. So although it is useful as a supplementary test for rabies diagnosis, it is not recommended as a primary diagnostic test12.
Rapid immunochromatographic diagnostic tests (RIDT) or lateral flow assays (LFA), especially in the field settings are useful for screening brain and saliva specimens of animals. These simple to perform tests require no expensive equipment, reagents, cold-chain facilities or technical expertise56,67,68,69,70,71. An LFA (Anigen Rapid Rabies Ag Test Kit, Bionote, Republic of Korea) was utilized for rapid post-mortem screening of rabies in dogs during routine surveillance in a study done in Goa. Compared to the gold-standard FAT, the test showed a sensitivity of 0.96 [95% confidence interval (CI): 0.91-0.99] and a specificity of 0.99 (95% CI: 0.94-1)68 which was similar to results reported by others67,69. Comparing various commercially available RIDTs to gold standard, however, reveals notable variations in sensitivity and specificity56,70,71. Results from preserved samples, even when frozen appropriately, may not be as accurate or ideal as fresh brain samples. Further validation studies are necessary to assess the reliability and performance of these assays for diagnosing rabies in humans.
Detection of virus-specific antibodies: The rapid fluorescent focus inhibition test or the fluorescent antibody virus neutralization test is used for the detection of neutralizing antibodies against RABV in CSF/and serum samples1,12,66. The performance of these tests is influenced by various factors, such as the growth of cell lines used, propagation and titration of laboratory-adapted RABV, sample quality (CSF and serum), the rabies conjugate used, availability of a functional fluorescent microscope and the technical expertise and skills of the analyst12,57,72. To enhance safety and reduce biohazard measures, pseudotype virus-based neutralization assays can be used instead of live RABV. These assays involve reporter genes and offer advantages such as lower sample volume requirements and reduced expenses, making them more feasible for laboratories with limited resources57,59,72,73,74.
The indirect fluorescent antibody (IFA) test is a simple and rapid test that can be used to detect IgM and IgG antibodies against RABV. However, its specificity is limited due to cross-reactivity with other viral encephalitis75. On the other hand, the ELISA can detect binding antibodies to glycoprotein and/or nucleoprotein, offering a safe, easy-to-perform and cost-effective alternative suitable for peripheral laboratories with basic facilities60,76,77.
Viral nucleic acid detection: Conventional or real-time reverse transcriptase (RT-PCR), nucleic acid sequence-based amplification, loop-mediated isothermal amplification and microarray-based assays are among the viral nucleic acid detection techniques for rabies1,12,66,73,78,79,80,81,82. The most popular method for confirming the diagnosis of rabies in a laboratory, in both ante-mortem and post-mortem samples is conventional or real-time RT-PCR. There are several standardized PCR protocols that target the nucleoprotein (N) gene or other rabies related genes40,41,42,43,61,66,73,83.
PCR exhibits higher sensitivity in pooled saliva and nuchal skin samples but lower sensitivity in CSF, urine samples and extracted hair follicles7,8,12,62,81,83,84,85. PCR techniques can be applied to decomposed, autolyzed and archived brain samples, whereas FAT may not yield reliable results with such samples1,8,12,66. These assays are suitable for samples stored in RNA solutions/buffers, as well as those collected and dried on filter/FTA paper and lateral flow device strips66,67,86.
A recent pilot study87 conducted in India evaluated a portable battery-operated chip-based real-time PCR assay (Truenat; Molbio Diagnostics) using animal brain samples. The study reported a sensitivity of 92.3 per cent, specificity of 100 per cent and diagnostic accuracy of 95.8 per cent, compared to FAT87. However, this assay has not yet been validated for use on human samples. Another portable real-time RT-PCR (PCR1100 assay) has recently been developed for rabies diagnosis and tested on animal samples, with 100 per cent reported sensitivity and specificity using brain tissues88. These rapid, user-friendly and cost-effective assays hold great potential for decentralizing laboratory services by eliminating the need for sample transportation. Such assays can be conveniently performed at the point of care, reducing the reliance on centralized laboratory facilities. This not only saves time but also minimizes the risk of sample degradation during transportation.
Virus isolation: Isolation of viable viruses can be achieved by rabies tissue culture infection test (RTCIT) or mouse inoculation test (MIT)1,12. The RTCIT can be performed on various sample types, including brain tissue, saliva and CSF. It involves use of specific cell lines such as murine neuroblastoma cells (Neuro-2a), human embryonic kidney cell line (HEK-293), chicken embryo-related cells or baby hamster kidney cells (BHK-21). These cell lines provide a suitable environment for the growth and replication of the virus, allowing its detection and isolation12,63,65,66. In the MIT, the virus is isolated through intracerebral inoculation of clinical samples into 3-4-week-old mice; the presence of virus can be detected by observing clinical signs and by laboratory testing of the infected tissues64,65,66.
However, both RTCIT and MIT have limitations and may not be practical as routine diagnostic techniques. MIT should be replaced with RTCIT in laboratories equipped with cell culture facilities whenever possible. Cell culture techniques offer several advantages, including avoidance of live animal use, cost-effectiveness and faster results.
Other diagnostic tests for rabies: Other potential diagnostic modalities for rabies include flow cytometry for demonstration of rabies viral antigen and antibodies89, mass spectrometry-based proteomics techniques for detection of rabies viral peptides90 and the utilization of biosensors82. However, for these tests to become standard-of-care assays, a thorough and extensive validation is necessary.
Interpretation of diagnostic test results: Several factors, including duration of illness, clinical presentation of the disease, intermittent shedding of the virus in clinical samples such as saliva, previous rabies vaccination and immune status of the patient, can influence the results of rabies diagnostic tests. Ante-mortem tests, such as virus isolation, viral antigen detection and viral RNA detection, are more likely to yield positive results during the early phase of the illness before neutralizing antibodies appear and cause the ‘autosterilization’ of tissues10.
Serological diagnosis, which involves detecting viral antibodies in CSF and/or serum, has limited utility due to the rapid and short clinical course of rabies in most affected individuals. The production of detectable levels of antibodies may occur later in the disease progression, making serological tests less reliable for early diagnosis. However, in individuals who survive beyond 1-2 weeks, serological tests can be valuable in confirming the diagnosis7,8,12.
An inverse correlation has been reported between the presence of neutralizing antibodies and the detection of viral RNA in clinical samples7,8,12,13,25,54. In addition, the individual vaccination status can also impact the results of PCR tests used for viral RNA detection. Those who had received at least partial rabies vaccination before the onset of illness have been shown to be less likely to have a positive PCR test result as compared to those unvaccinated7.
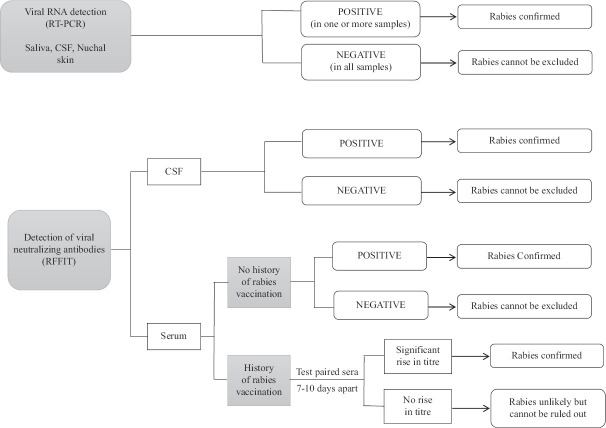
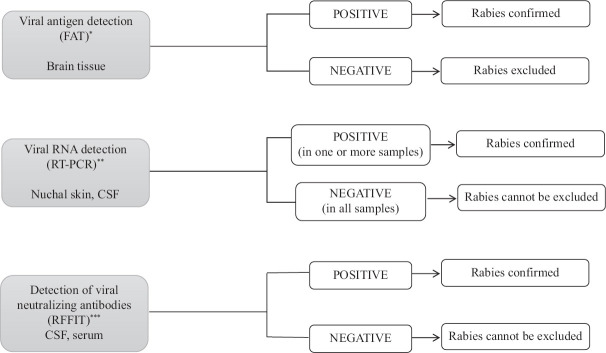
A diagnostic algorithm with recommended tests for ante-mortem and post-mortem laboratory confirmation of rabies12 is provided in Figs 1 and 2.
Fig 1.
Laboratory diagnosis of rabies (ante-mortem): testing algorithm. RFFIT, rapid fluorescent focus inhibition test. Note: Multiple sample types increase diagnostic sensitivity. If clinical suspicion is high in case of negative result, repeat testing.
Fig. 2.
Laboratory diagnosis of rabies (post-mortem): testing algorithm. FAT, Fluorescent antibody test. *RT-PCR can be used where FAT is not available. **Testing other samples recommended only if brain tissue not available. ***Post-mortem RFFIT is recommended only in individuals who have never received rabies vaccination.
Challenges in laboratory diagnosis of rabies
There is an urgent need for decentralization of rabies diagnostic facilities in India, as currently there are a limited number of laboratories involved in human rabies diagnosis, as listed in the NAPRE document35. To address this issue, the NRCP is working towards establishment of new rabies diagnostic facilities and enhancing the capabilities of existing laboratories. Moreover, laboratories set up during the COVID-19 pandemic can be repurposed for the diagnosis of other infectious diseases, including rabies, to optimize their utilization.
Ante-mortem laboratory testing for rabies involves several factors that can impact the results and interpretation, often requiring extensive or repeated testing to confirm the diagnosis. Two studies conducted at the Neurovirology laboratory at NIMHANS, Bengaluru, a WHO Rabies referral laboratory, audited clinical samples received from across India, highlighting the challenges associated with ante-mortem diagnosis. In these studies, ante-mortem laboratory confirmation of rabies was achieved in 40.6 per cent (128 cases between 2012 and 2014) and 42.3 per cent (130 cases between 2015 and 2017) of clinically suspected rabies cases7,8. The limited sensitivity of the tests was attributed to the absence of serial sampling and the inability to test multiple clinical samples. Due to the geographic distribution of cases, samples had to be transported over long distances to the laboratory, which posed challenges in maintaining the cold chain, influencing sample quality and test results. This issue is particularly significant in regions with high ambient temperatures throughout the year.
A major limitation of ante-mortem tests for rabies is that negative results do not exclude a diagnosis of the disease1,7,12. The gold standard for diagnosis is the testing of brain tissue obtained post-mortem, however, this poses major challenges due to logistical, religious, biosafety and other considerations.
In situations where autopsy or craniotomy for brain sample collection is not feasible, physicians should be aware that post-mortem brain biopsy can be obtained through alternative routes, ensuring that appropriate diagnostic options are considered to confirm or exclude a diagnosis of rabies. One approach is through the orbital or transnasal route using tru-cut biopsy needles. Another option is through the occipital route through the foramen magnum using lumbar puncture needles83,91.
Conclusion
Decentralization of laboratory facilities is a key strategy to improve access to diagnostic services for rabies in India. By strengthening laboratory infrastructure and expanding its scope, India can enhance public health infrastructure, improve infectious disease management and contribute significantly to the elimination of human rabies by 2030.
In addition to diagnostic functions, modern rabies laboratories should embrace a multidimensional role and actively integrate ‘One Health’ principles, contributing to the assessment, planning and investigation of rabies cases.
Financial support and sponsorship
None.
Conflicts of interest
None.
References
- 1.Pan American Health Organization (PAHO) World Health Organization. WHO expert consultation on rabies: Third report. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.World Health Organization. Adopt one health, stop rabies:India launches new national action plan for dog mediated rabies elimination by 2030. Geneva. 2021. [accessed on August 8, 2023]. Available from: https://www.who.int/news/item/25-10-2021-adopt-one-health-stop-rabies-india-launches-new-national-action-plan-for-dog-mediated-rabies-elimination-by-2030 .
- 3.Mitrabhakdi E, Wilde H, Hemachudha T. Rabies. In: Nath A, Berger JR, editors. Clinical neurovirology. New York: Marcel Dekker, Inc; 2003. pp. 309–26. [Google Scholar]
- 4.Warrell MJ, Warrell DA. Rabies: The clinical features, management and prevention of the classic zoonosis. Clin Med (Lond) 2015;15:78–81. doi: 10.7861/clinmedicine.14-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. Human rabies:Neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013;12:498–513. doi: 10.1016/S1474-4422(13)70038-3. [DOI] [PubMed] [Google Scholar]
- 6.Karande S, Muranjan M, Mani RS, Anand AM, Amoghimath R, Sankhe S, et al. Atypical rabies encephalitis in a six-year-old boy:Clinical, radiological, and laboratory findings. Int J Infect Dis. 2015;36:1–3. doi: 10.1016/j.ijid.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Damodar T, Mani RS, Prathyusha PV. Utility of rabies neutralizing antibody detection in cerebrospinal fluid and serum for ante-mortem diagnosis of human rabies. PLoS Negl Trop Dis. 2019;13:e0007128. doi: 10.1371/journal.pntd.0007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani RS, Anand AM, Madhusudana SN. Human rabies in India:An audit from a rabies diagnostic laboratory. Trop Med Int Health. 2016;21:556–63. doi: 10.1111/tmi.12669. [DOI] [PubMed] [Google Scholar]
- 9.Willoughby RE., Jr Rabies:Rare human infection –Common questions. Infect Dis Clin North Am. 2015;29:637–50. doi: 10.1016/j.idc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Jackson AC. Human disease. In: Fooks AR, Jackson AC, editors. Rabies scientific basis of the disease and its management. 4th ed. Oxford: Academic Press; 2020. pp. 277–302. [Google Scholar]
- 11.Mani RS, Willoughby RE. Human rabies in South Asia. In: Singh SK, editor. Neglected tropical diseases-South Asia. Neglected tropical diseases. New York: Springer; 2017. pp. 349–71. [Google Scholar]
- 12.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. An overview of antemortem and postmortem tests for diagnosis of human rabies; pp. 43–54. [Google Scholar]
- 13.Mani RS, Madhusudana SN. Laboratory diagnosis of human rabies:Recent advances. Scientific World Journal 2013. 2013:569712. doi: 10.1155/2013/569712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson AC. Therapy of human rabies. In: Fooks AR, Jackson AC, editors. Rabies scientific basis of the disease and its management. 4th ed. Oxford: Academic Press; 2020. pp. 547–66. [Google Scholar]
- 15.de Souza A, Madhusudana SN. Survival from rabies encephalitis. J Neurol Sci. 2014;339:8–14. doi: 10.1016/j.jns.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Netravathi M, Udani V, Rs M, Gadad V, Ashwini MA, Bhat M, et al. Unique clinical and imaging findings in a first ever documented PCR positive rabies survival patient:A case report. J Clin Virol. 2015;70:83–8. doi: 10.1016/j.jcv.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Manoj S, Mukherjee A, Johri S, Kumar KV. Recovery from rabies, a universally fatal disease. Mil Med Res. 2016;3:21. doi: 10.1186/s40779-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani RS, Damodar T, Divyashree S, Domala S, Gurung B, Jadhav V, et al. Case reports:Survival from rabies:Case series from India. Am J Trop Med Hyg. 2019;100:165–9. doi: 10.4269/ajtmh.18-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramaniam Mani R. Human rabies survivors in India:An emerging paradox? PLoS Negl Trop Dis. 2016;10:e0004774. doi: 10.1371/journal.pntd.0004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal K, Bhagwat C, Suthar R, Saini AG, Ravikumar N, Singh P, et al. Enigma of rabies:Prolonged survival in a boy with rabies brachial plexitis and encephalomyelitis. Neurol India. 2020;68:673–6. doi: 10.4103/0028-3886.288993. [DOI] [PubMed] [Google Scholar]
- 21.Madhusudana SN, Nagaraj D, Uday M, Ratnavalli E, Kumar MV. Partial recovery from rabies in a six-year-old girl. Int J Infect Dis. 2002;6:85–6. doi: 10.1016/s1201-9712(02)90144-x. [DOI] [PubMed] [Google Scholar]
- 22.Willoughby RE Jr., Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352:2508–14. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- 23.Hamide A, Kaliyappan A, Mani RS, Krishnamurthy A. Neurological recovery with serological response in a rabies survivor on long-term follow-up. Trop Doct. 2021;51:455–7. doi: 10.1177/0049475520983657. [DOI] [PubMed] [Google Scholar]
- 24.Karahocagil MK, Akdeniz H, Aylan O, Sünnetçioğlu M, Ün H, Yapici K, et al. Complete recovery from clinical rabies:Case report. Turk Klin J Med Sci. 2013;33:547–52. [Google Scholar]
- 25.Hunter M, Johnson N, Hedderwick S, McCaughey C, Lowry K, McConville J, et al. Immunovirological correlates in human rabies treated with therapeutic coma. J Med Virol. 2010;82:1255–65. doi: 10.1002/jmv.21785. [DOI] [PubMed] [Google Scholar]
- 26.Fooks AR, Cliquet F, Finke S, Freuling C, Hemachudha T, Mani RS, et al. Rabies. Nat Rev Dis Primers. 2017;3:17091. doi: 10.1038/nrdp.2017.91. [DOI] [PubMed] [Google Scholar]
- 27.Zeiler FA, Jackson AC. Critical appraisal of the Milwaukee protocol for rabies:This failed approach should be abandoned. Can J Neurol Sci. 2016;43:44–51. doi: 10.1017/cjn.2015.331. [DOI] [PubMed] [Google Scholar]
- 28.Maier T, Schwarting A, Mauer D, Ross RS, Martens A, Kliem V, et al. Management and outcomes after multiple corneal and solid organ transplantations from a donor infected with rabies virus. Clin Infect Dis. 2010;50:1112–9. doi: 10.1086/651267. [DOI] [PubMed] [Google Scholar]
- 29.Manesh A, Mani RS, Pichamuthu K, Jagannati M, Mathew V, Karthik R, et al. Case report:Failure of therapeutic coma in rabies encephalitis. Am J Trop Med Hyg. 2018;98:207–10. doi: 10.4269/ajtmh.17-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willoughby RE., Jr . Rabies treatment protocol and registry. Milwaukee (WI): Medical College of Wisconsin and Children's Hospital of Wisconsin; 2013. [accessed on August 8, 2023]. Available from: https://www.mcw.edu/rabies . [Google Scholar]
- 31.Lu XX, Zhu WY, Wu GZ. Rabies virus transmission via solid organs or tissue allotransplantation. Infect Dis Poverty. 2018;7:82. doi: 10.1186/s40249-018-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mrzljak A, Novak R, Pandak N, Tabain I, Franusic L, Barbic L, et al. Emerging and neglected zoonoses in transplant population. World J Transplant. 2020;10:47–63. doi: 10.5500/wjt.v10.i3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross RS, Wolters B, Hoffmann B, Geue L, Viazov S, Grüner N, et al. Instructive even after a decade:Complete results of initial virological diagnostics and re-evaluation of molecular data in the German rabies virus “outbreak” caused by transplantations. Int J Med Microbiol. 2015;305:636–43. doi: 10.1016/j.ijmm.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Zero by 30: the global strategic plan to end human deaths from dog-mediated rabies by 2030. [accessed on August 8, 2023]. Available from: https://www.who.int/publications/i/item/9789241513838 .
- 35.National Centre for Disease Control. National action plan for eliminating dog mediated rabies from India. National rabies control program. [accessed on August 8, 2023]. Available from: https://ncdc.gov.in/WriteReadData/linkimages/NationalActiopPlan.pdf .
- 36.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. The role of diagnostics in surveillance; pp. 35–42. [Google Scholar]
- 37.Brunker K, Nadin-Davis S, Biek R. Genomic sequencing, evolution and molecular epidemiology of rabies virus. Rev Sci Tech. 2018;37:401–8. doi: 10.20506/rst.37.2.2810. [DOI] [PubMed] [Google Scholar]
- 38.Nahata KD, Bollen N, Gill MS, Layan M, Bourhy H, Dellicour S, et al. On the use of phylogeographic inference to infer the dispersal history of rabies virus:A review study. Viruses. 2021;1628;13 doi: 10.3390/v13081628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes GJ, Smith JS, Hanlon CA, Rupprecht CE. Evaluation of a TaqMan PCR assay to detect rabies virus RNA:Influence of sequence variation and application to quantification of viral loads. J Clin Microbiol. 2004;42:299–306. doi: 10.1128/JCM.42.1.299-306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakeley PR, Johnson N, McElhinney LM, Marston D, Sawyer J, Fooks AR. Development of a real-time, TaqMan reverse transcription-PCR assay for detection and differentiation of lyssavirus genotypes 1, 5, and 6. J Clin Microbiol. 2005;43:2786–92. doi: 10.1128/JCM.43.6.2786-2792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wacharapluesadee S, Sutipanya J, Damrongwatanapokin S, Phumesin P, Chamnanpood P, Leowijuk C, et al. Development of a TaqMan real-time RT-PCR assay for the detection of rabies virus. J Virol Methods. 2008;151:317–20. doi: 10.1016/j.jviromet.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Nadin-Davis SA, Sheen M, Wandeler AI. Development of real-time reverse transcriptase polymerase chain reaction methods for human rabies diagnosis. J Med Virol. 2009;81:1484–97. doi: 10.1002/jmv.21547. [DOI] [PubMed] [Google Scholar]
- 43.Wadhwa A, Wilkins K, Gao J, Condori Condori RE, Gigante CM, Zhao H, et al. A pan-lyssavirus taqman real-time RT-PCR assay for the detection of highly variable rabies virus and other lyssaviruses. PLoS Negl Trop Dis. 2017;11:e0005258. doi: 10.1371/journal.pntd.0005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shipley R, Wright E, Selden D, Wu G, Aegerter J, Fooks AR, et al. Bats and viruses:Emergence of novel lyssaviruses and association of bats with viral zoonoses in the EU. Trop Med Infect Dis. 2019;4:31. doi: 10.3390/tropicalmed4010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunawardena PS, Marston DA, Ellis RJ, Wise EL, Karawita AC, Breed AC, et al. Lyssavirus in Indian flying foxes, Sri Lanka. Emerg Infect Dis. 2016;22:1456–9. doi: 10.3201/eid2208.151986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani RS, Dovih DP, Ashwini MA, Chattopadhyay B, Harsha PK, Garg KM, et al. Serological evidence of lyssavirus infection among bats in Nagaland, a North-Eastern state in India. Epidemiol Infect. 2017;145:1635–41. doi: 10.1017/S0950268817000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadin-Davis SA, Colville A, Trewby H, Biek R, Real L. Application of high-throughput sequencing to whole rabies viral genome characterisation and its use for phylogenetic re-evaluation of a raccoon strain incursion into the province of Ontario. Virus Res. 2017;232:123–33. doi: 10.1016/j.virusres.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marston DA, McElhinney LM, Ellis RJ, Horton DL, Wise EL, Leech SL, et al. Next generation sequencing of viral RNA genomes. BMC Genomics. 2013;14:444. doi: 10.1186/1471-2164-14-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rupprecht CE, Abela-Ridder B, Abila R, Amparo AC, Banyard A, Blanton J, et al. Towards rabies elimination in the Asia-Pacific region:From theory to practice. Biologicals. 2020;64:83–95. doi: 10.1016/j.biologicals.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Gibson AD, Yale G, Corfmat J, Appupillai M, Gigante CM, Lopes M, et al. Elimination of human rabies in Goa, India through an integrated one health approach. Nat Commun. 2022;2788;13 doi: 10.1038/s41467-022-30371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isloor S, Mani RS, Jayakrishnappa MB. Assessing rabies-free status of Andaman, Nicobar, and Lakshadweep Islands, India. Indian J Public Health. 2019;63:S48–50. doi: 10.4103/ijph.IJPH_412_19. [DOI] [PubMed] [Google Scholar]
- 52.Taib NAA, Labadin J, Piau P. Model simulation for the spread of rabies in Sarawak, Malaysia. Int J Adv Sci Eng Inf Technol. 2019;9:1739–45. [Google Scholar]
- 53.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. The direct fluorescent antibody test; pp. 108–29. [Google Scholar]
- 54.Mani RS, Madhusudana SN, Mahadevan A, Reddy V, Belludi AY, Shankar SK. Utility of real-time taqman PCR for antemortem and postmortem diagnosis of human rabies. J Med Virol. 2014;86:1804–12. doi: 10.1002/jmv.23814. [DOI] [PubMed] [Google Scholar]
- 55.Lembo T, Niezgoda M, Velasco-Villa A, Cleaveland S, Ernest E, Rupprecht CE. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg Infect Dis. 2006;12:310–3. doi: 10.3201/eid1202.050812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eggerbauer E, de Benedictis P, Hoffmann B, Mettenleiter TC, Schlottau K, Ngoepe EC, et al. Evaluation of six commercially available rapid immunochromatographic tests for the diagnosis of rabies in brain material. PLoS Negl Trop Dis. 2016;10:e0004776. doi: 10.1371/journal.pntd.0004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore SM, Goron CR. Measures of rabies immunity. In: Fooks AR, Jackson AC, editors. Rabies scientific basis of the disease and its management. 4th ed. Oxford: Academic Press; 2020. pp. 445–80. [Google Scholar]
- 58.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. The rapid fluorescent focus inhibition test; pp. 196–218. [Google Scholar]
- 59.Moore SM, Hanlon CA. Rabies-specific antibodies:Measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis. 2010;4:e595. doi: 10.1371/journal.pntd.0000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feyssaguet M, Dacheux L, Audry L, Compoint A, Morize JL, Blanchard I, et al. Multicenter comparative study of a new ELISA, PLATELIA RABIES II, for the detection and titration of anti-rabies glycoprotein antibodies and comparison with the rapid fluorescent focus inhibition test (RFFIT) on human samples from vaccinated and non-vaccinated people. Vaccine. 2007;25:2244–51. doi: 10.1016/j.vaccine.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Nagaraj T, Vasanth JP, Desai A, Kamat A, Madhusudana SN, Ravi V. Ante mortem diagnosis of human rabies using saliva samples:Comparison of real time and conventional RT-PCR techniques. J Clin Virol. 2006;36:17–23. doi: 10.1016/j.jcv.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Dacheux L, Reynes JM, Buchy P, Sivuth O, Diop BM, Rousset D, et al. A reliable diagnosis of human rabies based on analysis of skin biopsy specimens. Clin Infect Dis. 2008;47:1410–7. doi: 10.1086/592969. [DOI] [PubMed] [Google Scholar]
- 63.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. Virus isolation in cell culture:The rabies tissue culture infection test; pp. 85–95. [Google Scholar]
- 64.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. Virus isolation in animals:The mouse inoculation test; pp. 74–84. [Google Scholar]
- 65.National Centre for Disease Control. Directorate General of Health Services. Ministry of Health and Family Welfare. Government of India. Rabies general aspects & laboratory diagnostic techniques. 2022. [accessed on August 8, 2023]. Available from: https://ncdc.mohfw.gov.in/WriteReadData/linkimages/RabiesGeneral AspectsLaboratoryDiagnosticTechniques2022.pdf .
- 66.Lorraine M, McElhinney LM, Marston DA, Megan Golding M, Nadin-Davis SA. Laboratory diagnosis of rabies. In: Fooks AR, Jackson AC, editors. Rabies scientific basis of the disease and its management. 4th ed. Oxford: Academic Press; 2020. pp. 401–44. [Google Scholar]
- 67.Léchenne M, Naïssengar K, Lepelletier A, Alfaroukh IO, Bourhy H, Zinsstag J, et al. Validation of a rapid rabies diagnostic tool for field surveillance in developing countries. PLoS Negl Trop Dis. 2016;10:e0005010. doi: 10.1371/journal.pntd.0005010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yale G, Gibson AD, Mani RS, Harsha PK, Costa NC, Corfmat J, et al. Evaluation of an immunochromatographic assay as a canine rabies surveillance tool in Goa, India. Viruses. 2019;11:649. doi: 10.3390/v11070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tenzin T, Lhamo K, Rai PB, Tshering D, Jamtsho P, Namgyal J, et al. Evaluation of a rapid immunochromatographic test kit to the gold standard fluorescent antibody test for diagnosis of rabies in animals in Bhutan. BMC Vet Res. 2020;16:183. doi: 10.1186/s12917-020-02405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Servat A, Robardet E, Cliquet F. An inter-laboratory comparison to evaluate the technical performance of rabies diagnosis lateral flow assays. J Virol Methods. 2019;272:113702. doi: 10.1016/j.jviromet.2019.113702. [DOI] [PubMed] [Google Scholar]
- 71.Klein A, Fahrion A, Finke S, Eyngor M, Novak S, Yakobson B, et al. Further evidence of inadequate quality in lateral flow devices commercially offered for the diagnosis of rabies. Trop Med Infect Dis. 2020;5:13. doi: 10.3390/tropicalmed5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright E, Temperton NJ, Marston DA, McElhinney LM, Fooks AR, Weiss RA. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes:A cross-species comparison. J Gen Virol. 2008;89:2204–13. doi: 10.1099/vir.0.2008/000349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fooks AR, Johnson N, Freuling CM, Wakeley PR, Banyard AC, McElhinney LM, et al. Emerging technologies for the detection of rabies virus:Challenges and hopes in the 21st century. PLoS Negl Trop Dis. 2009;3:e530. doi: 10.1371/journal.pntd.0000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 1. Geneva: World Health Organization; 2018. Demonstration of lyssavirus antibodies by pseudotype virus micro-neutralization assays; pp. 252–8. [Google Scholar]
- 75.Rudd RJ, Appler KA, Wong SJ. Presence of cross-reactions with other viral encephalitides in the indirect fluorescent-antibody test for diagnosis of rabies. J Clin Microbiol. 2013;51:4079–82. doi: 10.1128/JCM.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welch RJ, Anderson BL, Litwin CM. An evaluation of two commercially available ELISAs and one in-house reference laboratory ELISA for the determination of human anti-rabies virus antibodies. J Med Microbiol. 2009;58:806–10. doi: 10.1099/jmm.0.006064-0. [DOI] [PubMed] [Google Scholar]
- 77.Realegeno S, Niezgoda M, Yager PA, Kumar A, Hoque L, Orciari L, et al. An ELISA-based method for detection of rabies virus nucleoprotein-specific antibodies in human antemortem samples. PLoS One. 2018;13:e0207009. doi: 10.1371/journal.pone.0207009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wacharapluesadee S, Hemachudha T. Nucleic-acid sequence based amplification in the rapid diagnosis of rabies. Lancet. 2001;358:892–3. doi: 10.1016/S0140-6736(01)06041-X. [DOI] [PubMed] [Google Scholar]
- 79.Sugiyama M, Ito N, Minamoto N. Isothermal amplification of rabies virus gene. J Vet Med Sci. 2003;65:1063–8. doi: 10.1292/jvms.65.1063. [DOI] [PubMed] [Google Scholar]
- 80.Muleya W, Namangala B, Mweene A, Zulu L, Fandamu P, Banda D, et al. Molecular epidemiology and a loop-mediated isothermal amplification method for diagnosis of infection with rabies virus in Zambia. Virus Res. 2012;163:160–8. doi: 10.1016/j.virusres.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Wacharapluesadee S, Hemachudha T. Ante- and post-mortem diagnosis of rabies using nucleic acid-amplification tests. Expert Rev Mol Diagn. 2010;10:207–18. doi: 10.1586/erm.09.85. [DOI] [PubMed] [Google Scholar]
- 82.Zandi M, Zandi S, Mohammadi R, Hosseini P, Teymouri S, Soltani S, et al. Biosensor as an alternative diagnostic method for rabies virus detection:A literature review. Biotechnol Appl Biochem. 2022;69:1348–53. doi: 10.1002/bab.2207. [DOI] [PubMed] [Google Scholar]
- 83.Dacheux L, Wacharapluesadee S, Hemachudha T, Meslin FX, Buchy P, Reynes JM, et al. More accurate insight into the incidence of human rabies in developing countries through validated laboratory techniques. PLoS Negl Trop Dis. 2010;4:e765. doi: 10.1371/journal.pntd.0000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wacharapluesadee S, Hemachudha T. Urine samples for rabies RNA detection in the diagnosis of rabies in humans. Clin Infect Dis. 2002;34:874–5. doi: 10.1086/338872. [DOI] [PubMed] [Google Scholar]
- 85.Hemachudha T, Wacharapluesadee S. Antemortem diagnosis of human rabies. Clin Infect Dis. 2004;39:1085–6. doi: 10.1086/423813. [DOI] [PubMed] [Google Scholar]
- 86.Nadin-Davis SA, Sheen M, Wandeler AI. Recent emergence of the Arctic rabies virus lineage. Virus Res. 2012;163:352–62. doi: 10.1016/j.virusres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 87.Abraham SS, Aparna S, Prathiush PR, Jose M. Chip based RT PCR test (TrueNat PCR) for peripheral level diagnosis of rabies in animals:A pilot study. IOSR J Agric Vet Sci. 2022;4:8–11. [Google Scholar]
- 88.Demetria C, Kimitsuki K, Yahiro T, Saito N, Hashimoto T, Khan S, et al. Evaluation of a real-time mobile PCR device (PCR 1100) for the detection of the rabies gene in field samples. Trop Med Health. 2023;51:17. doi: 10.1186/s41182-023-00501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vengatesan D, Raj GD, Raja A, Ramadass P, Gunaseelan L. Detection of rabies virus antigen or antibody using flow cytometry. Cytometry B Clin Cytom. 2006;70:335–43. doi: 10.1002/cyto.b.20104. [DOI] [PubMed] [Google Scholar]
- 90.Rupprecht CE, Fooks AR, Abela-Ridder B, editors. Laboratory techniques in rabies. 5th ed. Vol. 2. Geneva: World Health Organization; 2018. Mass spectrometry-based proteomic approaches for the detection of rabies virus peptides; pp. 183–94. [Google Scholar]
- 91.Mahadevan A, Suja MS, Mani RS, Shankar SK. Perspectives in diagnosis and treatment of rabies viral encephalitis:Insights from pathogenesis. Neurotherapeutics. 2016;13:477–92. doi: 10.1007/s13311-016-0452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]