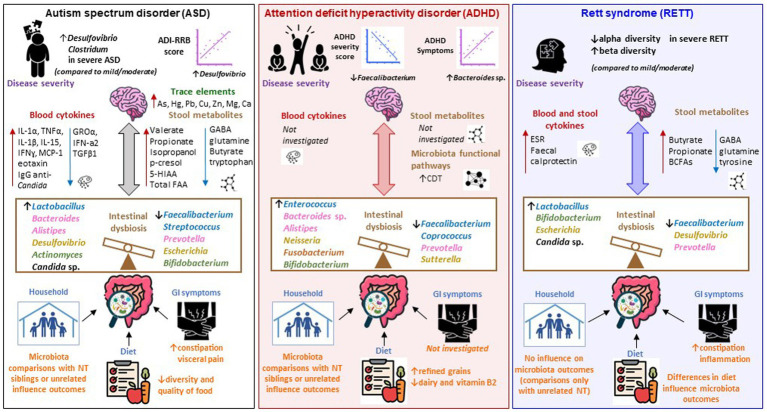
Figure 2.
Microbiota-gut-brain axis alterations in ASD, ADHD, and Rett syndrome. Schematic representation of the main findings emerged from the studies included in our systematic review, portraited as alterations across the microbiota-gut-brain axis in ASD (left panel), ADHD (middle panel), and Rett’s syndrome (RETT; right panel). Changes in the intestinal microbiota of subjects with ASD are associated with a high prevalence of GI symptoms and low-quality diet. The family household might have an influence in shaping gut microbiota communities, as ASD and family unrelated NT subjects have a different microbiota, however no differences are observed when ASD subjects are compared with their siblings. RETT subjects present intestinal inflammation (indicated by increased ESR and fecal calprotectin) and constipation and there are changes in the gut microbiota depending upon the type of diet (solid food vs. formula). No information is provided on the presence of GI disorders in ADHD subjects, however differences in dietary habits between ADHD subjects and NT controls have been shown even though their impact on the gut microbiota has not been investigated. Intestinal dysbiosis is mainly characterized by decreased Faecalibacterium (phylum Firmicutes) and Prevotella (phylum Bacteroides) in all three disorders. Increased Bacteroides sp. (phylum Bacteroidetes) were identified in ASD and ADHD. Lactobacillus (phylum Firmicutes) was increased in ASD and RETT. Bifidobacterium (phylum Actinobacteria) increased in ADHD and RETT and decreased in ASD. Desulfovibrio (phylum Proteobacteria) increased in ASD and decreased in RETT. Escherichia (phylum Proteobacteria) increased in RETT and decreased in ASD. Increased abundances of Candida sp. were identified whithin the mycobiota of ASD and RETT. Microbiota associated metabolites such as SCFAs and gut-brain neurotransmitters GABA, glutamine, tryptophan, and tyrosine are altered in ASD and RETT, however they have not been explored in ADHD studies. Increased p-cresol, isopropanol in the stool and trace elements As, Hg, Pb, Cu, Zn, Mg, Ca in the hair were found in ASD subjects. ASD is also associated with a dysregulation of the immune system (and potential inflammation), as blood cytokines IL-1α, TNFα, IL-1β, IL-15, IFNɣ, MCP-1, eotaxin are increased and GROα, IFN-a2, TGFβ1 are reduced. Conversely, no sign of inflammation was reported by studies investigating ADHD. Possible correlation between changes in microbial taxa and disease severity can be extrapolated across the three disorders. ASD severity is positively correlated with Desulfovibrio (phylum Proteobacteria). Hyperactivity and impulsivity (ADHD symptoms) are negatively correlated with Faecalibacterium (phylum Firmicutes) and positively correlated with Bacteroides sp. (phylum Bacteroidetes) in ADHD. A reduced alpha-diversity and increased beta diversity are associated with severe RETT (compared to mild/moderate). CDT was increased in the ADHD bacteriome. CDT is responsible of producing phenylalanine, an essential AA that crosses the BBB and acts as a precursor for dopamine and noradrenaline, key neurotransmitters involved in modulating behavior-related neuronal pathways in the brain. AA, amino acid; ADI-RRB, autism diagnostic interview-restricted and repetitive behaviors; ADHD, attention deficit hyperactivity disorder; ASD, Autism Spectrum Disorder; BBB, blood brain barrier; BCFAs, branched chain fatty acids; CDT, cyclohexadienyl dehydratase; GABA, Gamma-aminobutyric acid; GI, gastrointestinal; GROα, growth-related oncogene alpha; IL, Interleukin; IFN, Interferon; MCP-1, monocyte chemoattractant protein; NT, neurotypical controls; RETT, Rett syndrome; SCFAs, short chain fatty acids.