Fig. 1 |. Genetic models of mouse dendritic cell development and lineage restriction.
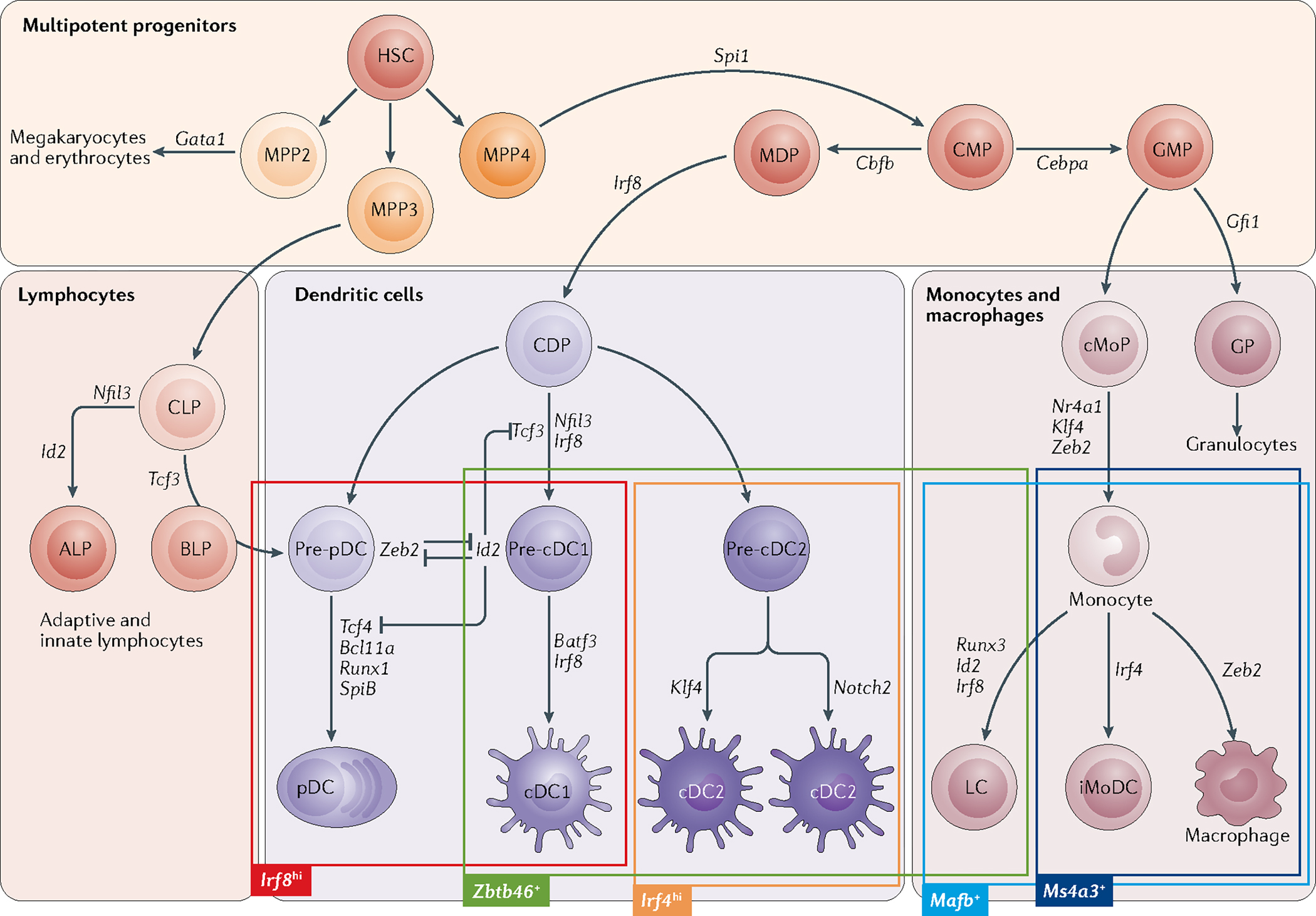
Hierarchical models of haematopoiesis are based on the developmental deficiencies that are observed in mice with mutations in transcription factor-encoding genes; these genes are shown adjacent to the associated stages of lineage restriction and specification. The figure highlights the development of mouse dendritic cell (DC), monocyte and macrophage subsets from shared progenitors. Haematopoietic stem cell (HSC)-derived multipotent progenitors (MPPs) undergo stages of differentiation to produce lineage-restricted progenitors of lymphocytes and myeloid cells — common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs). The CLP population can be separated into two subsets on the basis of Ly6D expression. The all-lymphoid progenitor (ALP) is Ly6D− and has B cell, T cell and innate lymphoid cell potential. The Tcf3-dependent B cell-biased lymphoid progenitor (BLP) is Ly6D+ and gives rise to B cells and plasmacytoid DCs (pDCs). High levels of expression of interferon regulatory factor 8 (Irf8) are associated with the specification of pDCs and type 1 classical DCs (cDC1s). Expression of Zbtb46 is a specific marker of cDC specification that is induced first in pre-cDC1s and precursor type 2 classical DCs (pre-cDC2s). cDC2 subsets are also characterized by high levels of Irf4 expression. Langerhans cells (LCs), monocytes and macrophages are all marked by Mafb-driven lineage tracing. Although LCs are not thought to be derived from the common DC precursor (CDP) population, given their embryonic origin, they do express Zbtb46 when they migrate to lymphoid organs from the skin. Lineage tracing based on Ms4a3 marks monocytes and monocyte-derived macrophages, as opposed to macrophages of embryonic origin. cMoP, common monocyte progenitor; GMP, granulocyte–macrophage progenitor; GP, granulocyte progenitor; iMoDC, immature monocyte-derived dendritic cell; MDP, monocyte–dendritic cell progenitor.
