Fig. 2 |. Stage-specific enhancer activation regulates Irf8-dependent specification of dendritic cell and monocyte progenitors.
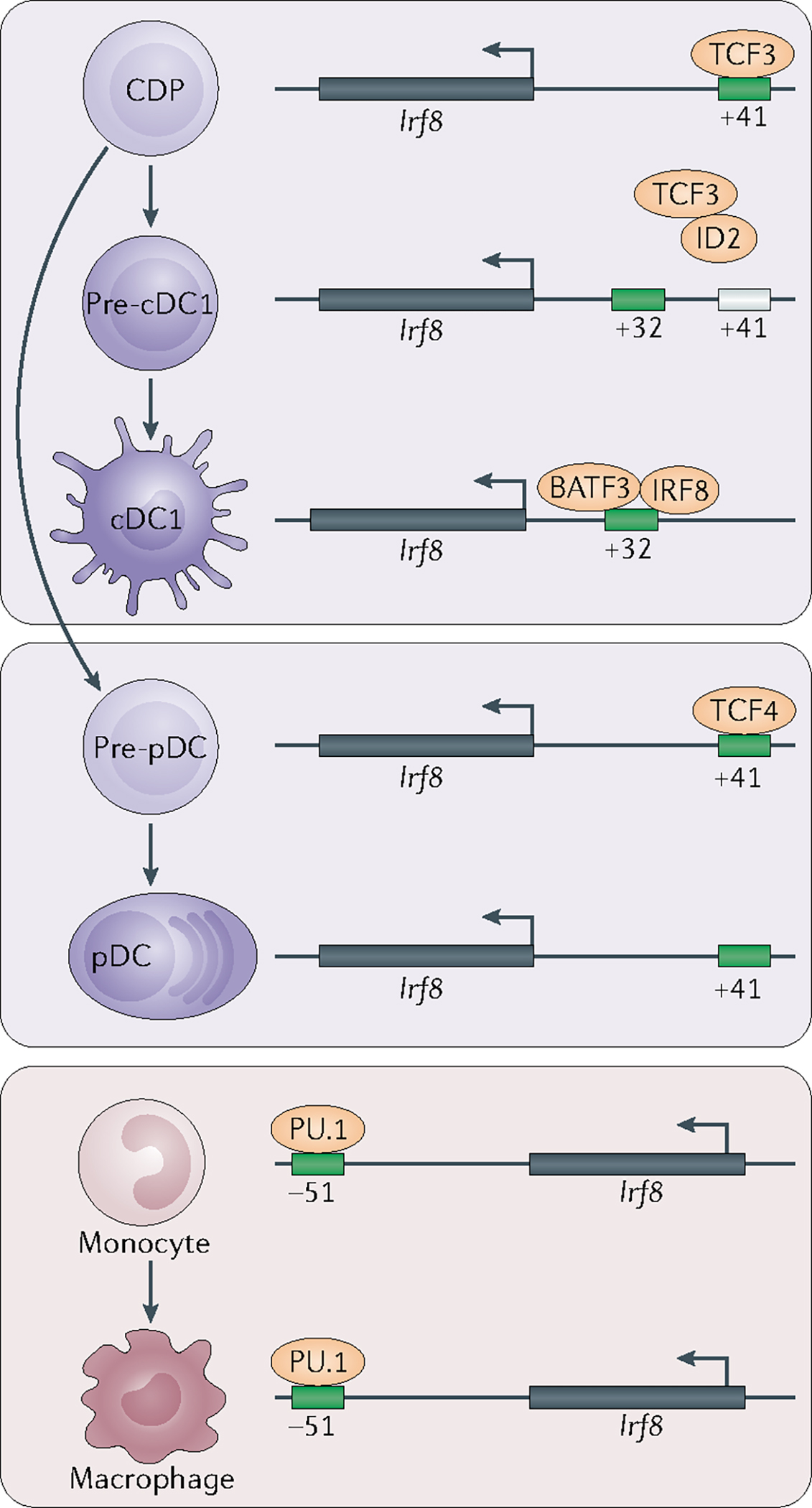
One mechanism for the development of type 1 classical dendritic cells (cDC1s) and plasmacytoid DCs (pDCs) from bone marrow progenitors is the strict regulation of interferon regulatory factor 8 (Irf8) expression by distal, evolutionarily conserved enhancer elements. E proteins — such as TCF3 (also known as E2A) and TCF4 (also known as E2–2) — support Irf8 expression through their actions at an E-box motif-containing enhancer located 41 kb upstream of the Irf8 transcription start site. An inactivating mutation at this locus impairs Irf8 expression in pDCs and blocks the specification of precursor cDC1s (pre-cDC1s) in the common DC precursor (CDP) population. On pre-cDC1 specification, Nfil3 is induced upstream of Id2. By blocking E protein activity, ID2 imposes a requirement for an alternative enhancer located 32kb upstream of Irf8 to promote Irf8 expression. In turn, Irf8 expression and cDC1 specification are supported by BATF3 and by IRF8-dependent autoactivation. An alternative enhancer located 51kb downstream of the Irf8 locus is required for Irf8 expression regulated by PU.1 in monocytes and macrophages.
