Abstract
Heterosexual transmission of a murine leukemia virus mixture named LP-BM5 MuLV, which is known as the murine AIDS virus, was investigated. Our results indicated that the heterosexual transmission of LP-BM5 MuLV occurs in both directions with high frequency and that the frequencies of virus transmission in the cervix and penis are higher than those in other genital organs. The results suggested that infection by LP-BM5 MuLV via heterosexual transmission may initially take place at particular retrovirus-sensitive sites (cells) in the genital organs.
Human immunodeficiency virus (HIV) infection is now pandemic. In many countries, HIV has been spread mainly by heterosexual transmission (3, 5). For the prevention of HIV infection, as well as for the development of vaccines against HIV, it is of a great importance to understand the mechanisms of the heterosexual transmission of retroviruses. Since it is difficult to investigate the mechanisms of heterosexual transmission of HIV in humans experimentally, an animal model with a retrovirus which induces an acquired immunodeficiency syndrome like human AIDS would be useful. A murine leukemia virus mixture called LP-BM5 MuLV induces a severe acquired immunodeficiency syndrome termed murine AIDS (MAIDS) in susceptible strains of mice (10). The mixture includes a replication-competent ecotropic virus, mink cell focus-inducing virus, and a replication-defective virus which has been considered to be involved in the pathogenesis of MAIDS (4). With many similarities to human AIDS patients, mice infected with the LP-BM5 MuLV mixture develop splenomegaly, systemic lymphadenopathy, and severe immunodeficiency (4, 11). We previously reported that maternal transmission of LP-BM5 MuLV occurs via mother’s milk with high frequency (12). In the present study, we demonstrate that the heterosexual transmission of LP-BM5 MuLV also occurs with high frequency via genital organs.
C57BL/10 (B10) mice were purchased from Japan SLC Inc., Shizuoka, Japan. All mice were specific-pathogen free and were housed in an air-conditioned room. They were given autoclaved water and sterilized pelleted feed. An SC-1 clone chronically infected with LP-BM5 MuLV, the G6 cell line, was kindly supplied by H. C. Morse III, National Institutes of Health, Bethesda, Md. Virus was prepared from the supernatant of G6 cells as previously described (12). The virus preparation was stored at −70°C until use. B10 mice were inoculated by the intraperitoneal route with 0.3 ml of the LP-BM5 MuLV preparation. To increase the frequency of sexual contacts and to avoid pregnancy in the female mice, all male mice were sterilized by vasectomy under anesthesia with pentobarbital (Nembutal). The vasectomized male mice were mated with female mice at least 4 weeks postoperation, since sperm are usually kept alive for 2 to 3 weeks in spermiducts. Excised genital organs were crushed with plastic sticks in 1 ml of lysis buffer containing 10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, 0.5% sodium dodecyl sulfate, and proteinase K (0.5 mg/ml). Spleen cells were lysed after hemolysis with 0.83% NH4Cl. Lysed samples were incubated at 50°C for 3 h. DNA was extracted three times with phenol-chloroform, precipitated with cold ethanol, treated with RNase and proteinase K, and dissolved in 0.1 ml of H2O. LP-BM5 MuLV defective virus genome was detected by Southern blot hybridization combined with PCR as described previously (12). In brief, template DNAs (1 μg per tube) were added to a cocktail adjusted to final concentrations of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, 200 μM deoxynucleoside triphosphate, 100 pmol of each primer (5′-CCTCTTCCTTTATCGACACT-3′ [sense] and 5′-ATTAGGGGGGGAATAGCTCG-3′ [antisense]), and 2 U of Taq DNA polymerase (Boehringer Mannheim) in a total volume of 100 μl and were subjected to 32 cycles of amplification. In each cycle of PCR, the mixture was denatured at 95°C for 1 min (5 min for the first cycle), annealed at 55°C for 3 min, and extended at 72°C for 1 min. The PCR-amplified products were subjected to gel electrophoresis (1.5% agarose) and transferred to a Hybond N+ membrane (Amersham) by the alkaline blotting method. Hybridization was achieved with a 5′ 32P-labeled probe (5′-TGTCAAAGGGACCAGTTAAG-3′) at 45°C overnight in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% sodium dodecyl sulfate–100 μg of salmon sperm DNA per ml. Hybridized membranes were washed twice in 2× SSC at 37°C for 10 min and then in 0.5× SSC at 45°C for 30 min. DNA derived from uterine cervices of uninfected mice was used as a negative control. The limit of sensitivity was approximately 10 copies per tube, as assessed by Southern blot analysis with plasmid DNAs (1/10 of the PCR product).
Concanavalin A (ConA) was obtained from Pharmacia Fine Chemicals, Uppsala, Sweden. Responder spleen cells (2 × 105) were cultured with ConA (5 μg/ml) in 96-well flat-bottomed microculture plates in 0.2 ml of culture medium at 37°C in 7.5% CO2. The culture medium consisted of RPMI 1640 supplemented with 10% fetal calf serum, penicillin (5,000 IU/100 ml), streptomycin (5,000 μg/100 ml), nonessential amino acids, sodium pyruvate (11.0 mg/100 ml), 2-mercaptoethanol (5 × 10−5 M), and l-glutamine (29.2 mg/100 ml). On day 2, cultures were pulsed with 1 μCi of [3H]thymidine and incubated for an additional 12 to 18 h. Incorporation of [3H]thymidine into responder spleen cells was quantitated by liquid scintillation counting. Determinations were performed in triplicate; standard errors of the means were generally <5% and therefore have not been indicated.
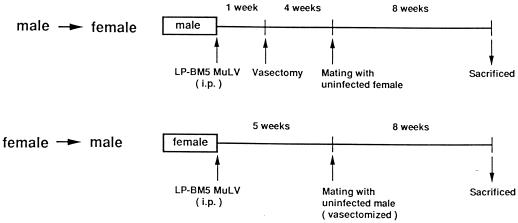
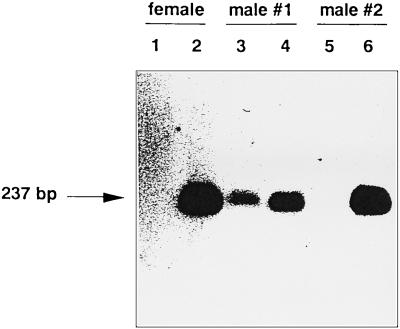
As illustrated in Fig. 1, in order to investigate the heterosexual transmission of LP-BM5 MuLV from male to female mice, normal male mice were inoculated with LP-BM5 MuLV and vasectomized 1 week later. At 5 weeks after virus inoculation, they were mated with uninfected female mice. After 8 weeks of breeding, female mice were sacrificed and their vaginae, cervices uteri, corpora uteri, inguinal lymph nodes, and spleens were removed and stored at −70°C until use. In the opposite direction, to investigate virus transmission from female to male, normal female mice were inoculated with LP-BM5 MuLV and then mated with uninfected, vasectomized male mice as described above. After 8 weeks of breeding, male mice were sacrificed and their penes, prepuces, inguinal lymph nodes and spleens were removed and stored at −70°C until use. Figure 2 shows the detection by PCR of the LP-BM5 defective virus genome in genital organs and spleens that were taken from mice mated with their virus-infected counterparts. It was demonstrated that although the defective virus genome was detected in both spleens and genital organs in some male mice (2 of 17 [see Table 1]), as shown in Fig. 2, lanes 3 and 4, the defective virus genome was detected only in the genital organs, not the spleens (Fig. 2, lanes 5 and 6), from most of the male mice. In contrast, all of the female mice were positive for defective virus genome only in the genital organs (Fig. 2, lanes 1 and 2). None of the mice examined were positive for the virus genome only in the spleens (this issue is discussed below). It should be noted here that the efficacy of PCR amplification, which was measured by experiments using the mixture of genomic DNA and plasmid DNA containing the defective virus, did not differ among the genital organs and spleens. By using the above strategy, the heterosexual transmission of LP-BM5 MuLV was investigated according to the protocol shown in Fig. 1.
FIG. 1.
Experimental design for examination of heterosexual transmission of the MAIDS virus in B10 mice. i.p., intraperitoneal.
FIG. 2.
Detection of the LP-BM5 MuLV defective virus genome by PCR in genital organs and spleens. The template DNAs (1 μg) derived from female or male mice which were bred with LP-BM5 MuLV-infected mice were amplified by PCR. Samples were prepared from either female (lanes 1 and 2) or male (lanes 3 to 6) mice. Lanes 1, 3, and 5, spleen; lane 2, uterine cervix; lanes 4 and 6, penis (from two representative male mice). The PCR products (5 μl) were applied to a 1.5% agarose gel and analyzed by Southern blotting with a probe for the defective virus (12).
TABLE 1.
Heterosexual transmission of LP-BM5 MuLV
| Expt | Clinical condition
|
Detection of LP-BM5 MuLV (no. positive/total [%])
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Spleen | Inguinal lymph node | Cervix | Corpus | Vagina | Penis | Prepuce | |
| 1 | MAIDS | Normal | 0/25 (0) | 0/16 (0) | 9/25 (36) | NDa | ND | ||
| 2 | MAIDS | Normal | 0/11 (0) | ND | 3/11 (27) | 0/11 (0) | 1/11 (9) | ||
| 3 | Normal | MAIDS | 1/8 (12) | 3/8 (38) | 6/8 (75) | 0/8 (0) | |||
| 4 | Normal | MAIDS | 1/9 (11) | ND | 5/9 (56) | 1/9 (11) | |||
ND, not done.
Twenty-five female mice that were mated with the virus-infected male mice were analyzed for the presence of LP-BM5 defective genome in their genital organs, lymph nodes, and spleens. As summarized in Table 1, the defective virus genome was detected with high frequency in cervices (9 of 25). However, the defective virus genome was not detected in spleens at all (0 of 25). The female genital organs are divided into three parts, namely, the vagina, cervix of uterus, and corpus of uterus. As also shown in Table 1, the cervix appears to be more sensitive to virus infection than the other organs. Since MAIDS virus was not detected in castrated female mice, which were kept with virus-infected male mice in the same cage, the virus infection occurred via heterosexual transmission rather than by nonheterosexual horizontal transmission (data not shown).
In 17 male mice mated with the virus-infected female mice (Table 1), the defective virus genome was detected in penes with high frequency (11 of 17). The defective virus genome was detected in DNA prepared from spleens with much lower frequency (2 of 17). In male mice, the penis seems to be much more sensitive to virus infection than are the prepuce and spleen (Table 1). In experiments 1 and 3, we also examined the inguinal lymph nodes from 16 female mice and 8 male mice. The defective virus genome was detected in some of the male mice (3 of 8) but not at all in the female mice examined. These results suggest that the LP-BM5 MuLV mixture initially infects the cervix or penis and then spreads over the whole body, including the lymph nodes and spleen.
To determine whether mice infected with LP-BM5 MuLV by heterosexual transmission in fact develop MAIDS, we examined both spleen weights and mitogen (ConA) responses of female mice at 10 months after mating. As shown in Table 2, female mice which were infected with LP-BM5 MuLV by heterosexual transmission (i.e., the defective virus genome was detected in the cervix) developed MAIDS as assessed by splenomegaly and decreased mitogen response, although the symptoms were less severe than of mice directly infected with LP-BM5 MuLV via the intraperitoneal route. Therefore, the cells in the genital organs were not only infected by the MAIDS virus but also able to replicate and spread the virus.
TABLE 2.
Development of MAIDS in heterosexually infected B10 mice
| Clinical condition
|
Spleen wt (mg) | Mitogen response (cpm) | Detection of LP-BM5 MuLV
|
||
|---|---|---|---|---|---|
| Male | Female | Spleen | Cervix | ||
| Normal | Normal | 105 | 39,981 | − | − |
| 92 | 19,317 | − | − | ||
| MAIDS | Normal | 136 | 10,346 | + | + |
| 186 | 7,799 | + | + | ||
| 245 | 4,911 | + | + | ||
The main route of HIV infection is heterosexual transmission (3, 5). However, the mechanisms of heterosexual transmission of retroviruses have been ill defined. HIV infection has been thought to occur during sexual contacts through slight injuries in the genital organs and to subsequently spread over the whole body. Among the genital organs of females, the parts of direct contact with male genital organs and semen are the vagina and cervix of uterus. The vagina is covered by a thick stratified squamous epithelium, while the cervix is covered by a monolayer columnar epithelium in addition to a squamous epithelium (2, 7). Histological examination (13) showed the presence of HIV-infected cells in the cervices derived from HIV carrier females (those infected with HIV by drug injections rather than by heterosexual transmission). Furthermore, a previous study utilizing female chimpanzees demonstrated that transmission of HIV could occur by insertion of cotton containing HIV into the vagina (8). These results suggested the presence of retrovirus-sensitive cells in genital organs. In our study, the cervix and penis are shown to be sensitive sites for virus infection (Table 1). Our assumption that there might be retrovirus-sensitive cells in a particular genital organ is currently under investigation by using in situ hybridization and immunohistochemical analyses.
The heterosexual LP-BM5 MuLV infection rate for females to males appeared to be higher than that for males to females (Table 1). The mating frequency of normal male mice with infected female mice is supposed to be higher than that of normal female mice with infected male mice, since normal female mice fall into false pregnancy after mating and therefore reject male mice for a few weeks. This difference may also be attributed to the longer retention of genital secretions containing LP-BM5 MuLV in the male genital organs because of their phimoses (9). In fact, the defective virus genome was detected in vaginal secretions (both in secreted fluid and cells) by PCR (data not shown). Alternatively, the penis might be a highly sensitive site for retrovirus infection. In this regard, it is interesting that the defective virus genome was detected with very low frequency (1 of 17 male mice) in the prepuce even though it is constantly in contact with the penis. It is worth mentioning that contamination by retroviruses in the seminal fluid may happen at the prostate, seminal vesicle, vas deferens, Cowper’s glands, or penile urethra, since the sterilized (vasectomized) mice were still capable of transmitting the viruses to female mice (1, 6).
The animal model for heterosexual transmission of retroviruses presented here has practical advantages, including (i) the high frequency of virus transmission and (ii) the possibility of rapid and cost-effective screening for antiretroviral agents (drugs and vaccines, etc.). This model may provide valuable information relating to heterosexual transmission of retroviruses including HIV and may further contribute to the prevention of HIV infection and the development of a remedy for AIDS.
Acknowledgments
We thank Junichiro Matsuda for teaching us the vasectomy procedure, Masahiko Makino for preparing the LP-BM5 MuLV mixture, and Sung-Tae Yee for critical review of the manuscript.
This work was partly supported by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Anderson D J, Politch J A, Martinez A, Van Voorhis B J, Padian N S, O’Brien T R. White blood cells and HIV-1 in semen from vasectomised seropositive men. Lancet. 1991;338:573–574. doi: 10.1016/0140-6736(91)91139-l. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein A, Sedlis A. Diseases of the vagina. In: Blaustein A, editor. Pathology of the female genital tract. 2nd ed. New York, N.Y: Springer-Verlag; 1982. pp. 59–98. [Google Scholar]
- 3.Cameron D W, Simonsen I N, D’Costa L J, Ronald A R, Maitha G M, Gakinya M N, Cheang M, Ndinya-Achola J O, Piot P, Brunham R C, Plummer F A. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet. 1989;ii:403–407. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay S K, Morse III H C, Makino M, Ruscetti S K, Hartley J W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci USA. 1989;86:3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clumeck N, Taelman H, Hermans P, Piot P, Schoumacher M, De Wit S. A cluster of HIV infection among heterosexual people without apparent risk factors. N Engl J Med. 1989;321:1460–1462. doi: 10.1056/NEJM198911233212107. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva M, Shevchuk M M, Cronin W J, Armenakas N A, Tannenbaum M, Fracchia J A, Ioachim H L. Detection of HIV-related protein in testes and prostates of patients with AIDS. Am J Clin Pathol. 1990;93:196–201. doi: 10.1093/ajcp/93.2.196. [DOI] [PubMed] [Google Scholar]
- 7.Frenczy A. Anatomy and histology of the cervix. In: Blaustein A, editor. Pathology of the female genital tract. 2nd ed. New York, N.Y: Springer-Verlag; 1982. pp. 119–135. [Google Scholar]
- 8.Fultz P N, McClure H M, Daugharty H, Brodie A, McGrath C R, Swenson B, Francis D P. Vaginal transmission of human immunodeficiency virus (HIV) to a chimpanzee. J Infect Dis. 1986;154:896–900. doi: 10.1093/infdis/154.5.896. [DOI] [PubMed] [Google Scholar]
- 9.Kreiss J K, Hopkins S G. The association between circumcision status and human immunodeficiency virus infection among homosexual men. J Infect Dis. 1993;168:1404–1408. doi: 10.1093/infdis/168.6.1404. [DOI] [PubMed] [Google Scholar]
- 10.Mosier D E. Animal models for retrovirus-induced immunodeficiency. Immunol Investig. 1986;15:233–261. doi: 10.3109/08820138609026687. [DOI] [PubMed] [Google Scholar]
- 11.Mosier D E, Yetter R A, Morse H C., III Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/10 mice. J Exp Med. 1985;161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada Y, Suzuki K, Komuro K, Mizuochi T. High frequency of transmission of murine AIDS virus in C57BL/10 mice via mother’s milk. J Virol. 1992;66:5177–5182. doi: 10.1128/jvi.66.9.5177-5182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomerantz R J, de la Monte S M, Donegan S P, Rota T R, Vogt M W, Craven D E, Hirsh M S. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108:321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]