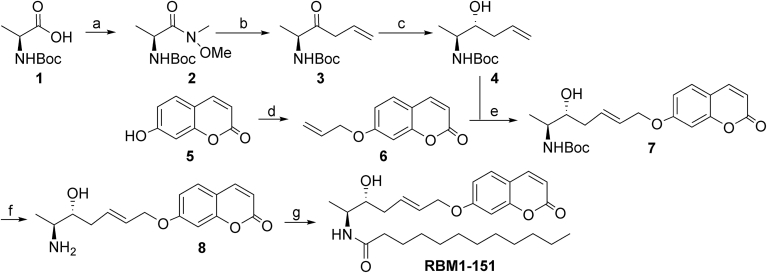
Scheme 2.
Synthesis of RBM1-151. Reagents and conditions: a) N,O-dimethylhydroxylamine (HCl), EDC·HCl, NMM, CH2Cl2, −15°C to room temperature, 4 h, 97%; b) Allylmagnesium bromide, THF, −78°C to room temperature, 3 h, 78%; c) LiAlH(OtBu)3, EtOH, −78°C to 0°C, 3 h, 77% (dr = 96:4); d) allyl bromide, acetone, reflux; e) second G Grubbs catalyst, CH2Cl2, 6 h, reflux, 50%; f) CH3COCl, MeOH, 24 h, 88%; g i) NEt3, CH2Cl2,ii) lauric acid, HOBt, EDC·HCl, CH2Cl2, 70%.