Figure 1.
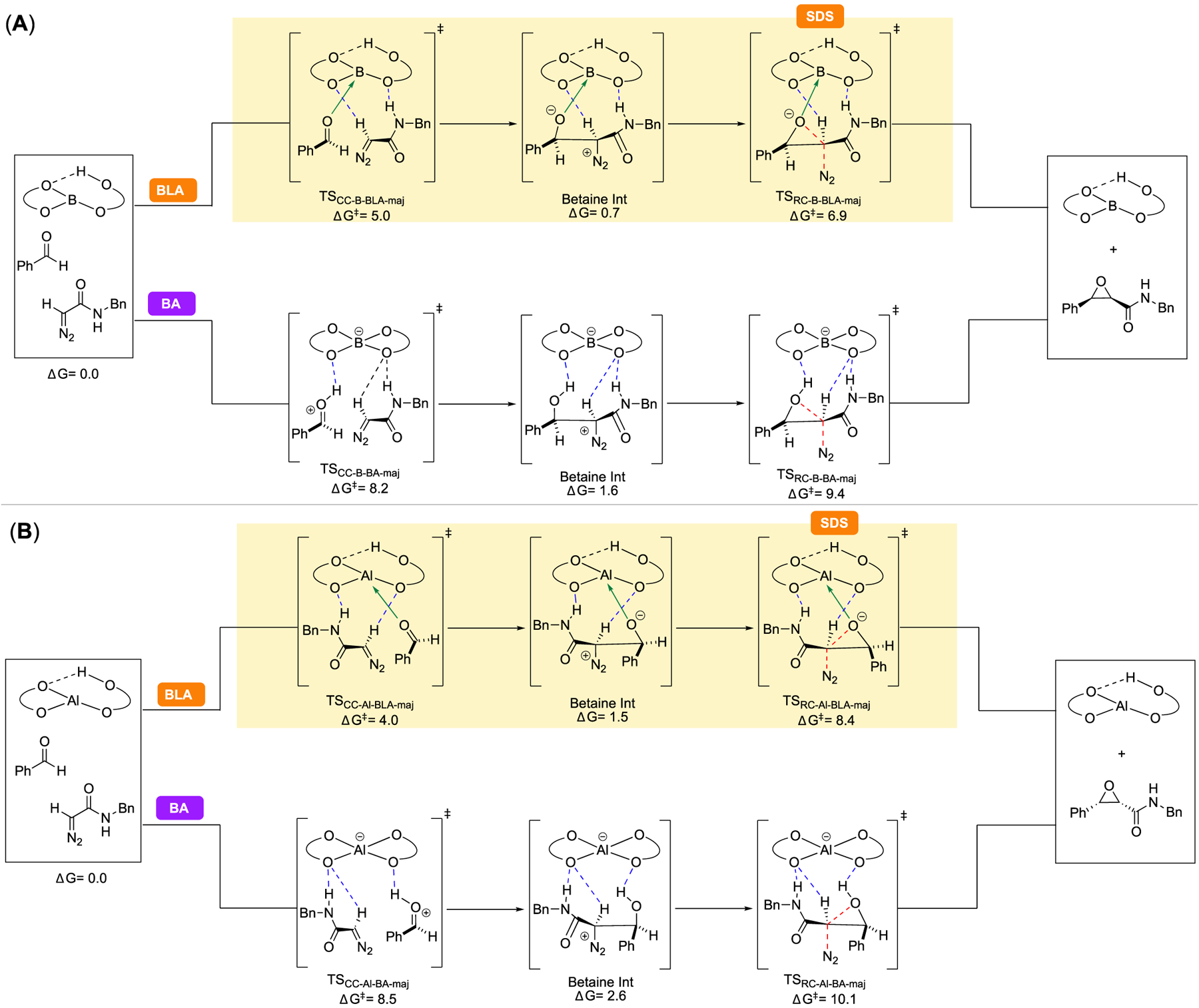
Free energies of key stationary points in the reaction coordinate leading to the major enantiomer of cis-epoxide 24a for the reaction catalyzed by the (A) (R)-VANOL boron-catalyst and (B) (R)-VANOL aluminum-catalyst, computed at M06–2x/6–311++G** PCM (toluene)//B3LYP-D3/6–31G*. Both Brønsted acid-assisted Lewis acid (BLA) and Brønsted acid (BA) pathways are shown, and the reported energies for each transition state and intermediate are relative to the prereactive complex of the respective catalyst with benzaldehyde (18) and diazoacetamide 1a in the BLA pathway, which is the lowest-energy stationary point in either pathway for both catalysts. The preferred pathway for each catalyst is highlighted in yellow, and the selectivity-determining step (SDS) is indicated.
