Table 2.
Epoxidation of Aromatic Aldehydes with VANOL and VAPOL Aluminum Catalystsa
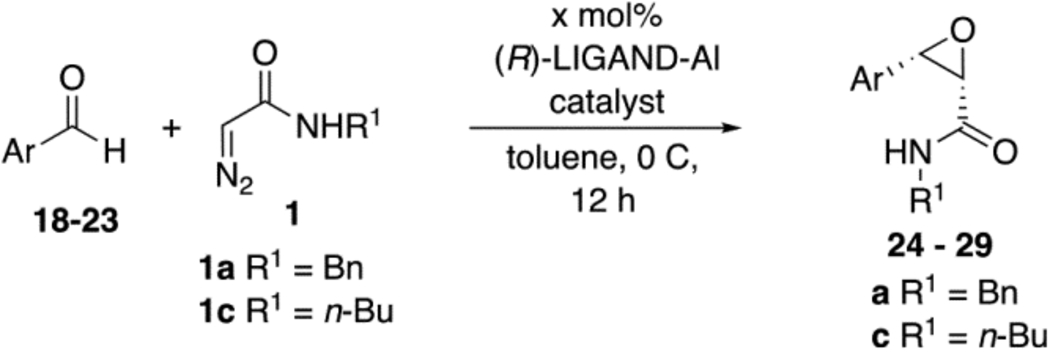
| ||||||
|---|---|---|---|---|---|---|
| aldehyde | epoxide | Ligand | catalyst (mol%) | R1 | % yield epoxideb | %ee epoxidec |
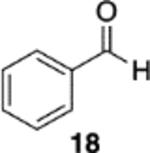
|
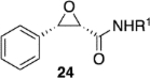
|
(S)-VAPOL | 5 | Bn | 97 | −99d |
| (S)-VAPOL | 5 | n-Bu | 86 | −97d | ||
| (S)-VAPOL | 10 | Bn | 84 | −99d | ||
| (S)-VANOL | 10 | Bn | 89 | −81d | ||
| (S)-VANOL | 10 | n-Bu | 19e,f | nd | ||
| (S)-VANOL | 5 | n-Bu | 86g | 93 | ||
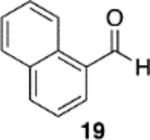
|
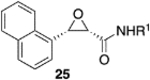
|
(S)-VAPOL | 5 | Bn | 93 | −99d |
|
|
(S)-VAPOL | 5 | Bn | 76 | −99d |
| (S)-VAPOL | 5 | n-Bu | 66 | −93d | ||
|
|
(S)-VAPOL | 10 | Bn | 11e,h | nd |
|
|
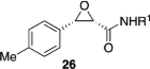
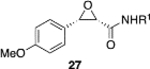
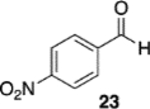
(S)-VAPOL | 5 | Bn | 90 | 99d |
| (S)-VAPOL | 5 | n-Bu | 77 | −85d | ||
|
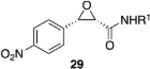
|
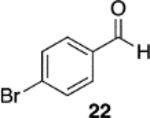
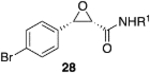
(S)-VAPOL | 5 | Bn | 55 | −62d |
| (S)-VAPOL | 5 | n- Bu | 36 | −71d | ||
| (R)-VAPOL | 10 | Bn | 70 | 93 | ||
Unless otherwise specified, all reactions were carried out in toluene at 0 °C for 12 h with 0.5 mmol diazo compound 1 at 0.1 M with 1.2 equiv of aldehyde. The catalyst was prepared as indicated in Table 1.
Isolated yields.
Determined by HPLC. nd = not determined.
The enantiomer of the epoxide was formed.
NMR yield.
This reaction as carried out at −40 °C for 12 h.
This reaction was with the boron catalyst prepared as in Scheme 2 without DMSO and was performed at −40 °C for 2 h. See ref 1.
Reaction went to 50% conversion and also gave a 10% yield of a β-keto amide.
