FIGURE 1.
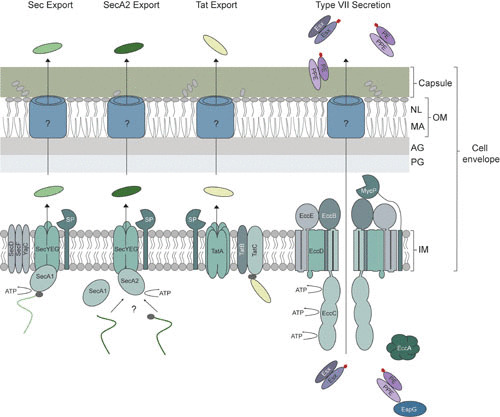
Model of the mycobacterial cell envelope and export systems. Although the mycobacterial cell envelope contains a traditional cytosolic (inner) membrane, the peptidoglycan layer is covalently linked to an arabinogalactan layer consisting of arabinose and galactose, which in turn is covalently linked to mycolic acids. These unusually long fatty acids (up to 90 carbon atoms) are one of the main components of the outer membrane, which also contains noncovalently bound (free) lipids. The final layer of the cell envelope is the capsule, mainly consisting of polysaccharides and proteins. The cell envelope is highly impermeable and unique to other Gram-positive bacteria. To export proteins into and across the cell envelope, mycobacteria have four systems, Sec, SecA2, Tat, and type VII secretion (T7S). The Sec pathway exports unfolded proteins across the inner membrane, and the substrates bind to an ATPase, SecA1, which targets the substrates to the translocation channel consisting of SecYEG and provides energy for translocation. The additional membrane components SecD, SecF, and YajC increase the efficiency of export. Upon translocation across the inner membrane, the N-terminal signal peptide (depicted in gray) is removed by a signal peptidase (SP). Less is known about the SecA2 pathway. Substrates are dependent on the ATPase SecA2; however, studies show that they also utilize the SecYEG channel and possibly SecA1, as well. The list of SecA2-dependent exported proteins includes examples with and without a signal peptide. The third export system, the Tat pathway, exports folded proteins. The Tat substrates, containing an N-terminal signal peptide, with a pair of arginine residues, is targeted to the membrane components TatBC, which then recruit homo-oligomers of TatA for subsequent transportation across the membrane. Similar to Sec, the signal peptide is removed by a signal peptidase. The T7S system consists of five conserved membrane components, of which EccBCDE form the secretion complex. EccC is the ATPase, providing the required energy for the secretion process. Although the mycosin protease (MycP) is not an integral component of the secretion complex, it associates with the complex and is essential for successful secretion. The substrates (Esx and PE-PPE) are secreted as heterodimers and are targeted to the secretion complex by a conserved secretion signal that includes a YxxxD/E motif (depicted in red), whereas the cytosolic chaperone EspG is required for directing the PE-PPE pairs to their specific T7S. The role of the second conserved cytosolic component, EccA, remains uncertain. It is currently unknown how Sec, SecA2, Tat, and T7S substrates are secreted across the outer membrane into the capsular layer or culture supernatant. IM, inner membrane; PG, peptidoglycan layer; AG, arabinogalactan layer; MA, mycolic acids; NL, noncovalently bound lipids; OM, outer membrane.
