FIGURE 5.
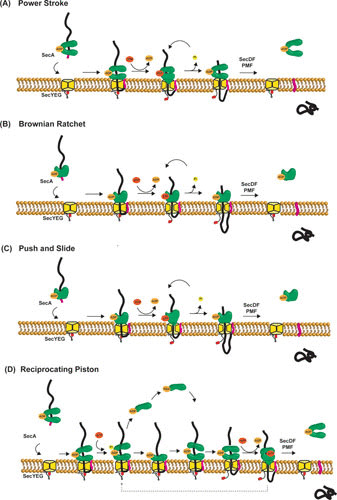
Proposed models of SecA-mediated protein translocation. (A) Power stroke: ATP binding and hydrolysis induce conformational changes of SecA that result in a mechanical force on the preprotein, pushing it through the SecYEG channel. In this model, oligomerization of SecA is required to prevent backsliding of the preprotein. (B) Brownian ratchet: SecA regulates the SecYEG channel opening via the 2HF of SecA, allowing the protein translocation via diffusion. Trapping and release of the translocating preprotein at the cis-side result in translocation, while SecA may fulfill an additional function by opening the translocation channel. The oligomeric state of SecA is not explicitly shown in this model. (C) Push and slide: this model uses both SecA-dependent pushing and Brownian motion. The oligomeric state of SecA is not explicitly shown in this model. (D) Reciprocating piston: this model is a combination of a power stroke mechanism with the conversion of dimeric-monomeric SecA. Repeated cycles of SecA monomerization-rebinding and ATP binding-hydrolysis yield a stepwise translocation process. In none of these models is the exact role of the PMF and SecDF included, but they contribute to efficient translocation.
