FIGURE 5.
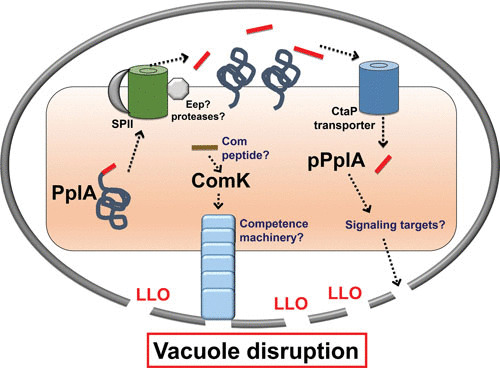
L. monocytogenes peptide signaling and expression of competence gene products via phage excision within the host vacuole. pplA encodes a lipoprotein (PplA) with a peptide pheromone (pPplA) located within the N terminal secretion signal peptide (shown in red). The signal sequence of prePplA is processed by signal peptidase II (SPII), and the released signal peptide is further cleaved by the protease Eep, releasing the pPplA pheromone, while the PplA protein becomes lipid modified and associated with the membrane. The confined space of the vacuole leads to import of the secreted pPplA pheromone, presumably stimulating a signaling cascade that results in the production of an unknown factor(s) that contributes to vacuole lysis. As part of a separate pathway, an unknown signal, potentially also involving a peptide pheromone, leads to the expression of ComK and the expression of a competence-associated pilus that may also aid in vacuole membrane disruption. Select L. monocytogenes comK genes harbor a lysogenic phage that must excise to enable ComK expression. The ComK pathway is required for L. monocytogenes vacuole escape from professional phagocytic cells; the pPplA system is required for bacterial escape from nonprofessional phagocytic cells.
