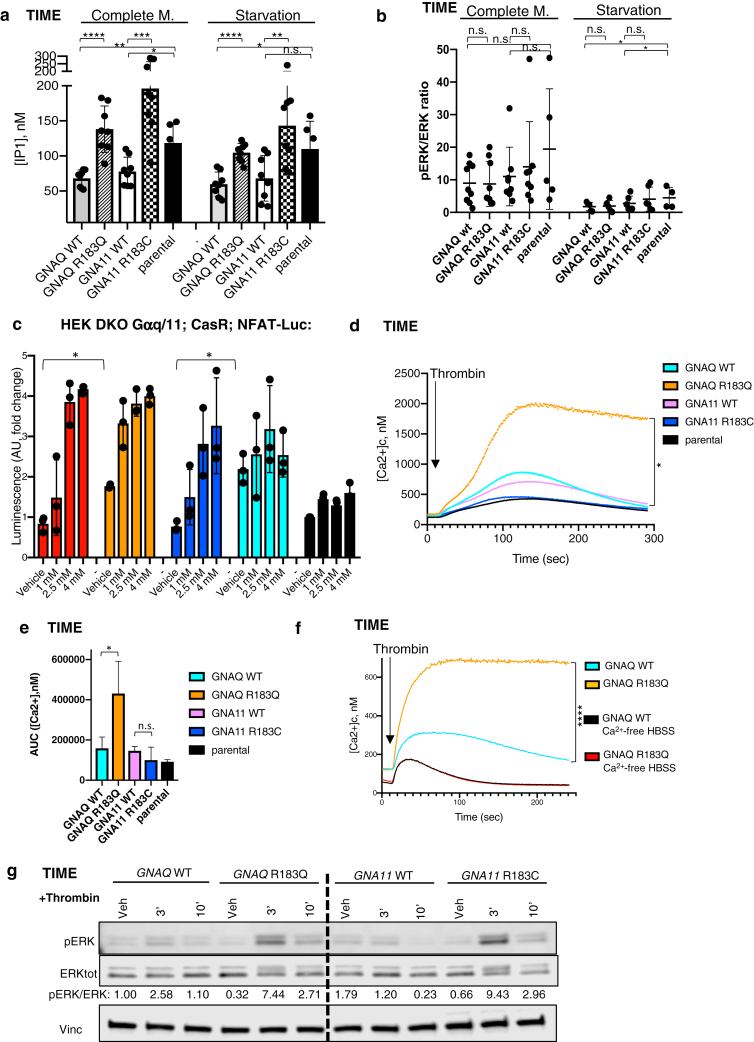
Figure 1.
Effect of GNAQ/GNA11 variants on constitutive and ligand-induced intracellular calcium and ERK signaling in endothelial cells. (a) TIME recombinant or parental cell lines were assayed for concentration of IP1 in complete medium and starvation conditions. The graph represents the mean ± SD of 8 independent experiments. Statistical comparison among conditions was by two-tailed unpaired t-test (∗∗∗∗P < .0001, ∗∗∗P = .0003, ∗∗GNA11WT versus GNA11R183CP = .0059, ∗∗GNA11WT versus parental P = .0025, ∗P < .05, n.s. = nonsignificant). (b) Densitometric analysis performed on a minimum of five independent western blot experiments on TIME cell lines in complete medium or after 1-hour acute starvation. Results are shown as mean ± SD. Two-tailed unpaired t-tests did not reveal statistically significant differences between GNAQ or GNA11 WT and variant cell lines in any condition (n.s. = nonsignificant, ∗P GNAQWT versus parental starvation = .048, ∗P GNA11WT versus parental starvation = .032). (c) HEK DKO Gaq/11; CaSR;NFAT-Luc cells were transfected with the GNAQWT, GNAQR183Q, GNA11WT, or GNA11R183C constructs and treated with vehicle or three concentrations of extracellular calcium to stimulate activation of CaSR and downstream G-protein signaling. Luciferase activity was measured 4 hours after stimulation. The graph represents the mean ± SD of three independent experiments. Statistical comparison among different conditions was performed by two-tailed paired t-test (∗ P < .05). (d) TIME recombinant or parental cell lines were loaded with intracellular calcium probe Fluo-8 and stimulated with thrombin (1U/Ml) in HBSS standard buffer. Changes in fluorescence over the time were recorded and normalised to maximum and minimum responses to calculate cytosolic (Ca2+). The graph represents an average of three independent experiments performed with four technical replicates. Statistical test comparing GNAQR183Q and GNAQWT is described in (e). (e) Means ± SD deviation of areas under the curve calculated from three experiments summarised in Figure 1d. Statistical comparisons were performed by two-tailed unpaired t-test (n.s. = statistically nonsignificant, ∗ P = .049). (f) TIME-GNAQWT or GNAQR183Q were loaded with intracellular calcium probe Fluo-8 and stimulated with thrombin (1U/Ml) in HBSS standard buffer (yellow and blue lines) or after 100-second-long exposure to HBSS calcium-free buffer (black and red lines). Changes in fluorescence over the time were recorded and normalised to maximum and minimum responses to calculate cytosolic Ca2+ level. The graph represents an average of three independent experiments performed with six technical replicates. Statistical test performed by two-way ANOVA (∗∗∗∗P <.0001). (g) Western blot time-course analysis of TIME recombinant cell lines starved for 1 hour and treated by vehicle or thrombin (1U/Ml) for the times indicated. Lysates were probed with the indicated antibodies. Densitometric quantification of pERK/ERK bands showed increased activation of the pathway in both GNAQ and GNA11 variant cells compared with WT counterparts following 3′ or 10′ treatment by thrombin. One representative of three independent experiments is shown. AUC, area under curve; ERK, extracellular signal–regulated kinase; HBSS, Hanks' Balanced Salt Solution; HEK, human embryonic kidney; IP1, inositol-1-phosphate; n.s., nonsignificant; pERK, phosphorylated ERK; TIME, telomerase-immortalised microvascular endothelial; WT, wild type.