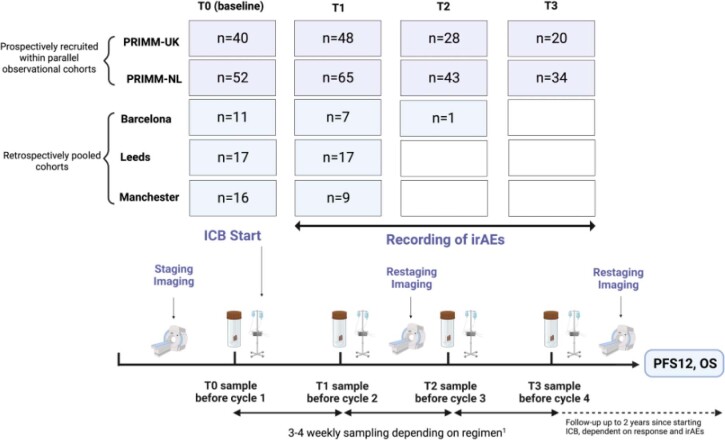
Extended Data Fig. 1. Study description with sample numbers across study visits.
Samples were collected within 5 sub-cohorts: two prospectively recruited within parallel observational studies (The PRIMM cohorts), and three retrospectively pooled cohorts. Fecal samples were collected at 4 timepoints: at baseline (T0) and at every treatment cycle (T1 to T3) over a period of 12 weeks. The time between two samples was 3 or 4 weeks, depending on the treatment regimen, with Ipilimumab/Nivolumab combination therapy and Pembrolizumab monotherapy administered 3-weekly and Nivolumab monotherapy administered 4-weekly. Treatment continued after the 12 weeks until the patient responded or until the treatment had to pause/stop due to irAEs. Not all subjects provided fecal samples at all study visits. Therefore, gut microbial dynamics were modeled at the level of the population including a random effect for the patient identifier (see Methods). Sample numbers represent patients with complete metadata (that is, no missingness) for all considered covariates/confounders. For the survival analysis, because we adjusted for a smaller number of covariates/confounders, there were n = 147 at baseline (PRIMM-UK = 41; PRIMM-NL = 53; Barcelona = 12; Leeds = 17; Manchester = 24) rather than n = 136 as indicated here. Tumor staging by CT or PET-scans was performed at study entry and at regular intervals during treatment. Tumor response was classified using the Response Evaluation Criteria in Solid Tumors (RECIST) v.1. Endpoints were defined as Progression-free survival at 12 months (PFS12) and overall survival (OS). Immune-related adverse events (irAEs) were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v.5 (see Table 1). ICB, Immune checkpoint blockade; PRIMM, Predicting Response to Immunotherapy for Melanoma With Gut Microbiome and Metabolomics; NL, Netherland; UK, United Kingdom. The figure was generated in BioRender.com.