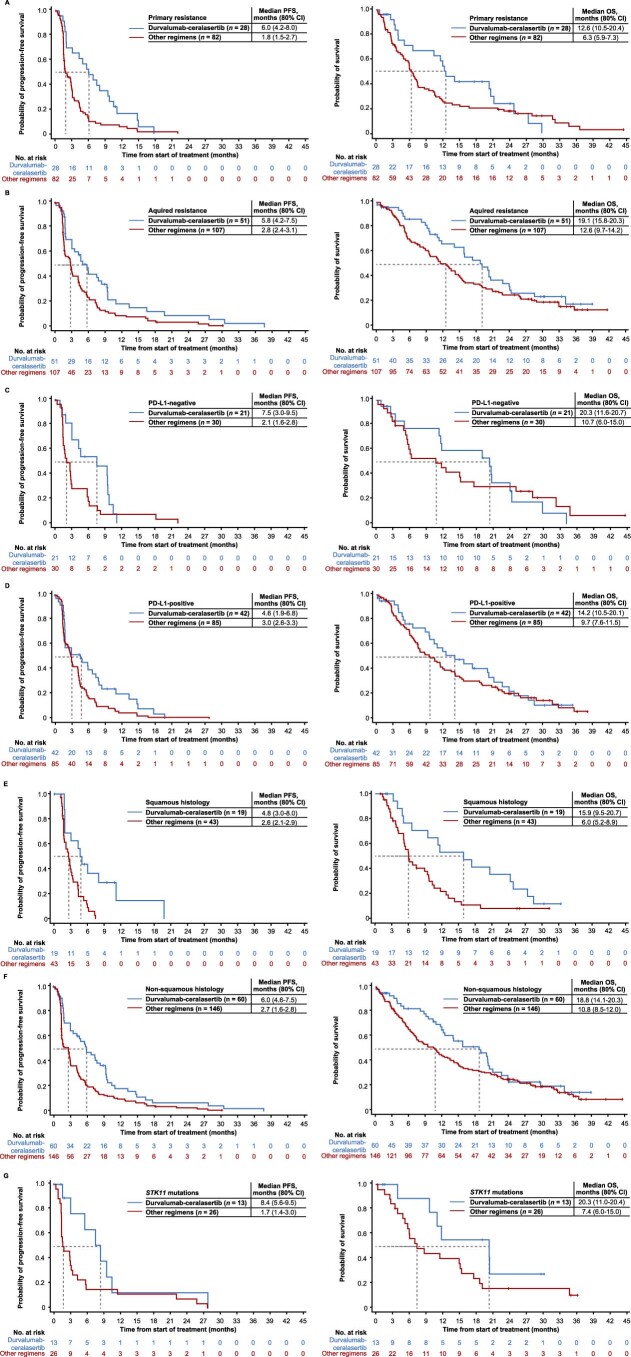
Extended Data Fig. 3. PFS and OS with durvalumab-ceralasertib and with durvalumab plus olaparib, danvatirsen or oleclumab in patient subgroups defined by presence or absence of adverse prognostic factors.
Kaplan–Meier analyses of (left) PFS and (right) OS in patients with a, primary resistance, b, acquired resistance, c, PD-L1-negative tumours, d, PD-L1-positive tumours, e, squamous histology, f, non-squamous histology, and g, STK11 mutations.