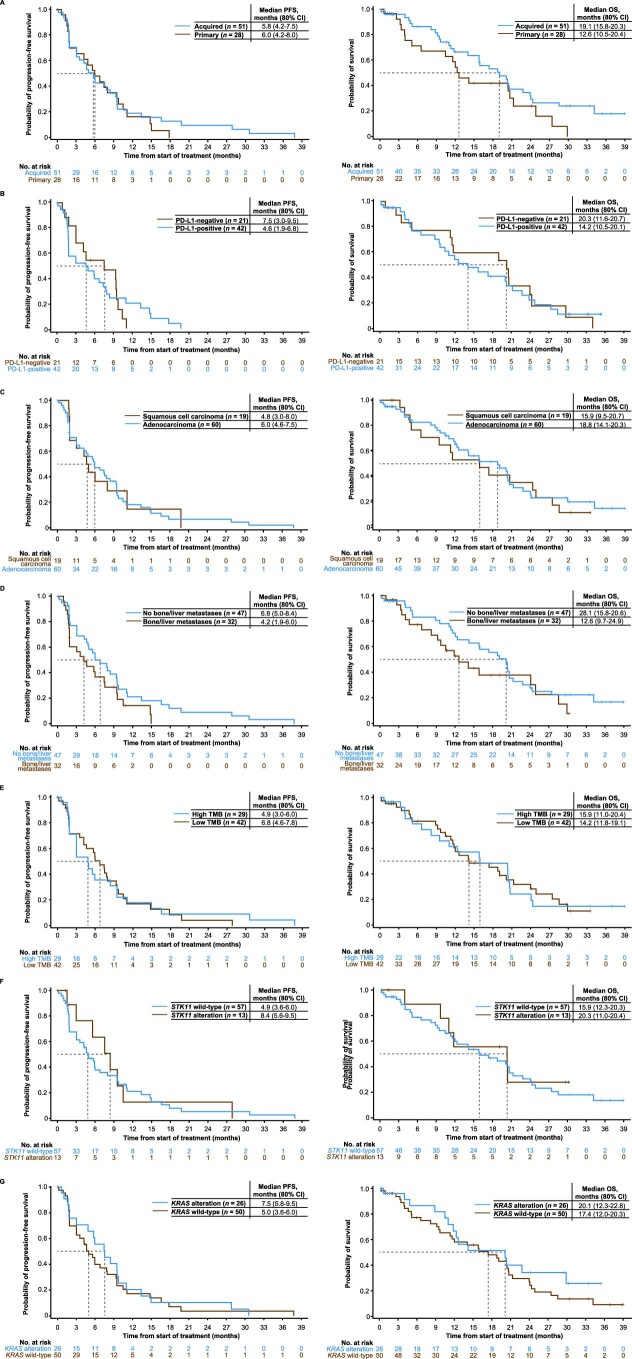
Extended Data Fig. 4. PFS and OS with durvalumab-ceralasertib in patient subgroups defined by presence or absence of adverse prognostic factors.
Kaplan–Meier analyses of (left) PFS and (right) OS with durvalumab-ceralasertib in patients with a, primary vs acquired resistance, b, PD-L1-negative vs -positive status, and c, squamous cell carcinoma vs non-squamous histology, and in patients d, with or without bone/liver metastases, e, with high or low tumour mutational burden, and according to f, STK11 status and g, KRAS status (variant or wild-type).