Fig. 1 |. Cellular sources, target cells, signalling and downstream effects of IL-12.
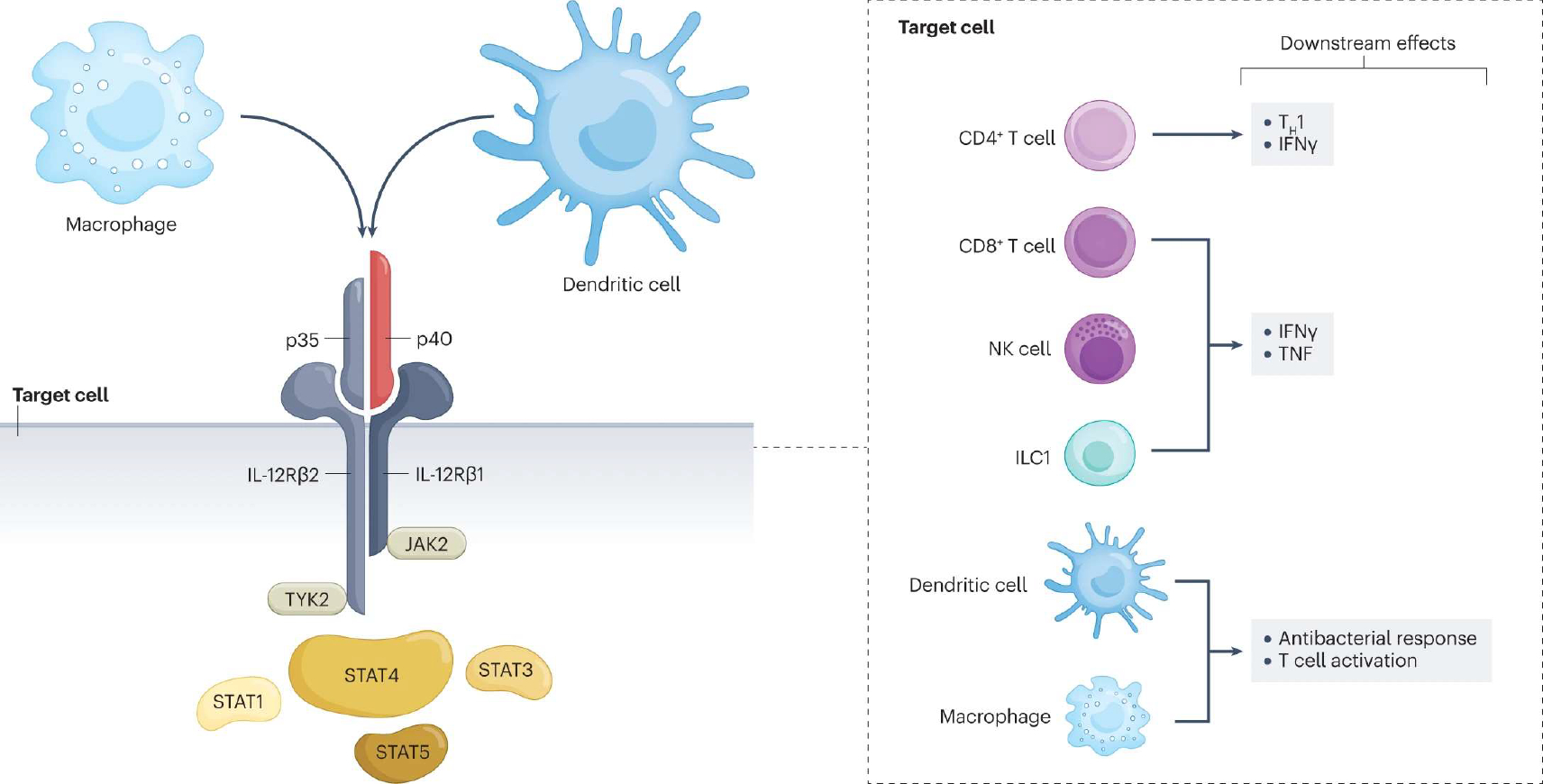
Interleukin-12 (IL-12) is a heterodimeric cytokine comprised of p40 (also part of the IL-23 dimer) and p35 subunits. It is produced by macrophages and dendritic cells. The receptor for IL-12 is composed of two different subunits, IL-12Rβ1 and IL-12Rβ2, which undergo conformational changes upon binding to IL-12 and bring into proximity two cytoplasmic tyrosine kinases, the Janus kinase 2 (JAK2) and tyrosine kinase 2 (TYK2), which are essential for downstream signalling of IL-12. JAKs trans/autophosphorylate each other and the receptor. Receptor phosphorylation enables binding and phosphorylation of signal transducers and activators of transcription (STATs), mainly STAT4. Phosphorylated STATs dimerize and translocate to the nucleus, where they regulate gene transcription. Different cell types express the IL-12 receptor on their membrane and are therefore targets for IL-12. Depending on the cell target, IL-12 exerts a variety of downstream effects. In naive CD4+ T cells, STAT4 signalling together with T-bet induce differentiation towards the T helper 1 (TH1) cell phenotype and production of interferon-γ (IFNγ). In CD8+ T cells, natural killer (NK) cells and group 1 innate lymphoid cells (ILC1s), IL-12 induces IFNγ and tumour necrosis factor (TNF) release. Finally, IL-12 signalling on dendritic cells and macrophages amplifies the antibacterial response and T cell activation.
