Fig. 2 |. Cellular sources, target cells, signalling and downstream effects of IL-23.
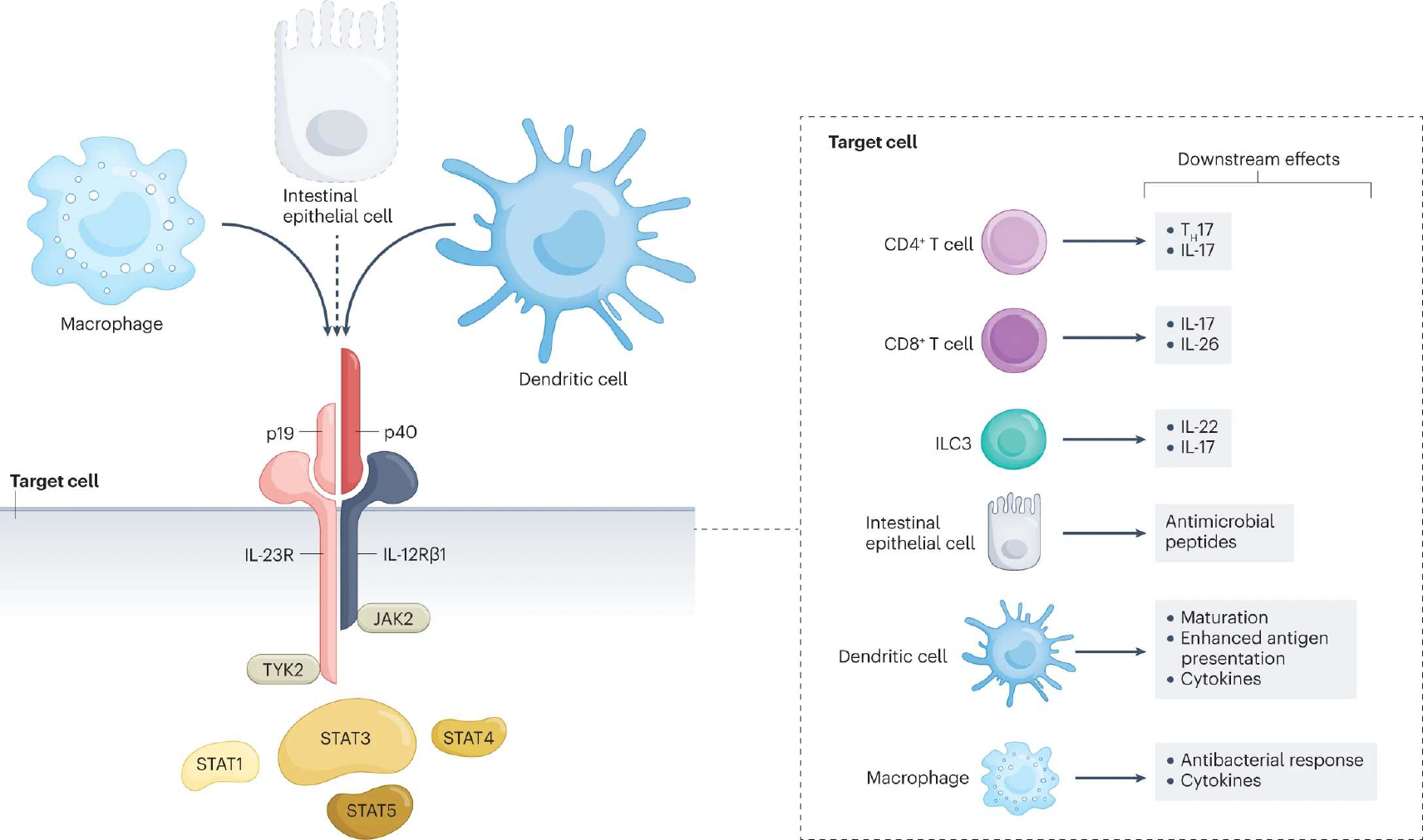
Interleukin-23 (IL-23) is a heterodimeric cytokine composed of p40 (also part of the IL-12 dimer) and p19 subunits. Like IL-12, and in response to similar stimuli (microbial signals, cytokines and co-stimulatory T cell ligands), IL-23 is produced by macrophages and dendritic cells. Some publications5–7 also show production of IL-23 by intestinal epithelial cells (dashed line, will need further confirmatory evidence). The IL-23 receptor is comprised of the IL-12Rβ1 and the IL-23R chains. Binding of IL-23 induces a conformational change that brings two cytoplasmic tyrosine kinases, Janus kinase 2 (JAK2) and tyrosine kinase 2 (TYK2), into proximity. JAKs trans/autophosphorylate each other and the receptor. Receptor phosphorylation enables binding and phosphorylation of signal transducers and activators of transcription (STATs), predominantly the STAT3 transcription factor. Phosphorylated STATs dimerize and translocate to the nucleus, where they regulate gene transcription. The IL-23 receptor is expressed on numerous cell types, including lymphoid cells, specifically CD4+ T helper 17 (TH17) and CD8+ T cells8. Innate lymphoid cells (ILCs), such as ILC3, also express the receptor and readily produce IL-22 and IL-17 cytokines in response to IL-23 stimulation. In IECs, IL-23 induces the expression of antimicrobial peptides. Furthermore, dendritic cells and macrophages respond to IL-23 stimulation by secreting a variety of cytokines. In addition, when the IL-23 receptor is engaged, dendritic cells demonstrate enhanced maturation and antigen presentation, and macrophages show an increased antibacterial response.
