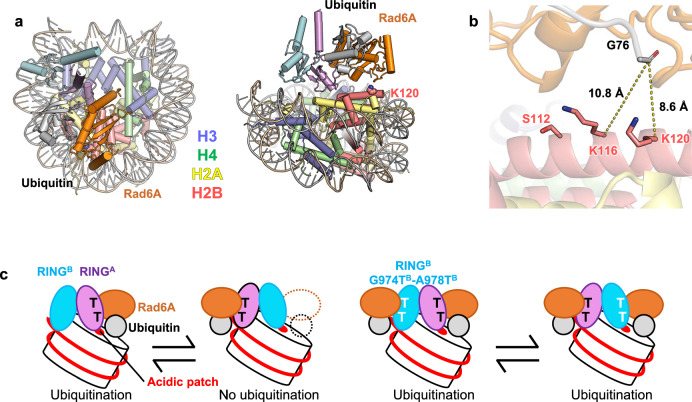
Fig. 5. A model for H2BK120-specific ubiquitination by the Bre1 complex.
a Model structure of the RINGA-RINGB-Rad6A-ubiquitin complex bound to the nucleosome in two views. b Close-up view of ubiquitin and H2B. Two lysine residues (H2BK120 and H2BK116) near G76 of ubiquitin are shown. H2BS112, whose GlcNAcylation stimulates H2BK120 ubiquitination, is also shown. c Proposed mechanistic model. The wild-type Bre1 complex can bind to the nucleosome in two orientations, but H2BK120 ubiquitination occurs only when Bre1A binds to the acidic patch, as RINGA, but not RINGB, can recruit Rad6A and ubiquitin. Bre1B with G974TB-A978TB double substitution can recruit Rad6A and ubiquitin; thus, H2BK120 ubiquitination occurs in both binding modes.