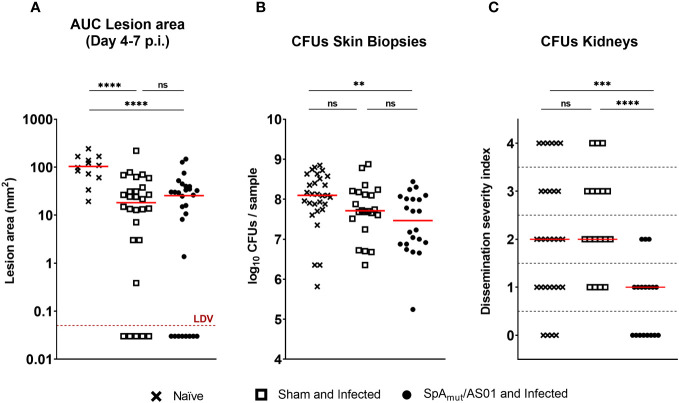
Figure 2.
Vaccination with SpAmut/AS01 protects against local manifestations and systemic sequelae in a skin infection recurrence mouse model of S. aureus infection. Skin (A, B) and systemic (C) readouts used to assess protection of SpAmut/AS01 vaccine in the skin infection recurrence model. (A) Area under the curve (AUC) indicated as mm2 of dermonecrotic lesion developed from day 4 to day 7 after skin infection recurrence. The red dotted line represents the Lower Detectable Value (LDV). ****p val< 0.0001. (B) Log10 of colony-forming unit (CFU) counts enumerated in homogenized skin biopsies collected 7 days after skin infection recurrence. **p val< 0.01; n.s. p val > 0.05. (C) Dissemination severity index assigned to single mice based on CFU counts recovered in the kidneys of infected mice 7 days after skin infection recurrence. Score 0, no bacteria; Score 1, very low dissemination (1–10 CFU/kidneys); Score 2, minimal dissemination (11–100 CFU/kidneys); Score 3, mild dissemination (101–1,000 CFU/kidneys); Score 4 severe dissemination (>1,001 CFU/kidneys). ***p val< 0.001; ****p val< 0.0001; n.s. p val > 0.05. For all the graphs above, each single dot represented data from a single animal, and red lines were median values of the groups. A total of 29 animals belong to the age-paired naïve mice (Naïve) group, 18 animals make up the group of mice not vaccinated but exposed to S. aureus during the first infection (sham and infected), and 18 animals belong to the group of mice vaccinated and infected (SpAmut/AS01 and Infected). The Kruskal–Wallis and uncorrected Dunn’s post-test was used to assess significance among groups.